CLIA Final Rule
Your Risk-Free IQCP Inspection Tips
CAP has released a set of instructions for inspectors on how to assess laboratory IQCPs. I will be honest: it's mostly toothless.
 Your Risk-Free IQCP Inspection Tips
Your Risk-Free IQCP Inspection Tips
Sten Westgard, MS
January 2016
As the new year unfolded before us, the various accreditation organizations have been unveiling their IQCP implementations. CAP has been helpful in not only providing a set of summary forms that condense a laboratory's IQCP into a standard format, but also giving a transparent view on how their inspectors will examine and assess an IQCP.
Unfortunately, those inspection standards are, to put it mildly, lenient. Laboratories evaluating their risk of being cited for serious IQCP deficiencies should consider this risk very low, infrequent if not improbable.
See if you understand why by glancing at the tips below...
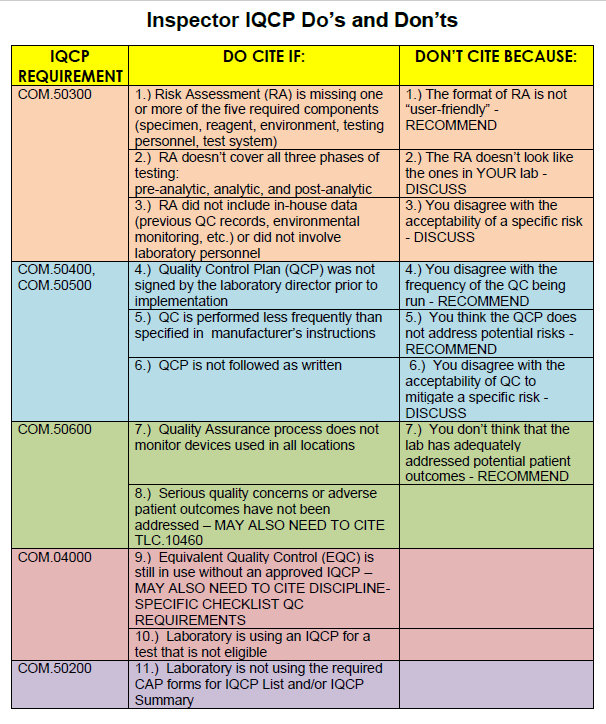
Cite-worthy offenses mainly come down to formatting and structure: did the lab include the 5 components of the Risk Assessment? Did they look at pre-analytical, analytical, and post-analytical phases of the testing process? Did the Director sign the Quality Control Plan? This is about paperwork and document headings and ticking the boxes. There's a list of error sources that are found itnhe CMS training guideline - as long as you hit all the points on that list, you've hit minimum compliance.
What's more concerning is what inspectors can't cite. If the inspector thinks the lab has underestimated the risks of the test, they can't stop the lab, they can only discuss it. If the inspector believes a risk posed by the test is actually unacceptable, the laboratory can't be cited (again, all that can be done is "discuss" the issue). If the inspector believes the lab should be running QC more frequently, the laboratory can't be cited, the inspector can only recommend more robust efforts. If the inspector believes the QCP doesn't address some of the risks of the test, the laboratory can't be cited, the inspector can only recommend additional quality control planning. If the inspector believes that the current QCP won't mitigate the risks of the test, the laboratory can't be cited.
In other words, an inspector may believe the laboratory has an inferior QCP, but the laboratory cannot be cited for it. There's almost no floor on low your IQCP can go. If you do a terrible job with your IQCP, but it's in the right format, you've filled out the right forms, you've pretended to address all the required error sources, and you've got the signatures, at best you're going to get a "discussion" and a "recommendation." As long as you've convinced yourself that you've done the right amount of Risk Assessment, CAP isn't ultimately able to disagree with you.
CAP inspectors have one card to play: they can cite the lab if "serious quality concerns or adverse patient outcomes haven't been addressed." There's a lot of leeway there both ways. The inspector can point out something that hasn't been included in the IQCP. But the laboratory can counter that it isn't a serious quality concern.
To be fair, CAP inspectors had the same problem with their EQC inspections. Inspectors weren't allowed to disagree with the laboratory if the director decided one device was "Option 1" and QC only needed to be done once a month, while the inspector felt it was really an "Option 2" device and QC should really be performed once a week. The laboratory director's decision trumped the inspector. That precedent has carried over as the EQC regulations are replaced by IQCP regulations. The subjective judgments of the laboratory director about risk acceptability and the ability of the quality control plan to mitigate those risks are infallible. One may wonder, if this is the case, why have an inspector at all? If the inspector's judgment isn't trusted, and they can't actually make any meaningful citations of poor practices, why have them evaluate the IQCP?
We can't blame CAP for this paradox, this is inspection as theater, since the root cause really lies with CMS. CAP is still the best accreditation provider not only in the US, but probably the world. Their checklists remain some of the most comprehensive and useful guidelines to good laboratory practice. CMS, however, with its ability to deem and un-deem, forces CAP to accept any weakening of regulations that it wants. So as the regulations on quality are loosened, the inspectors still need to go through the motions, because neither CMS nor the CAP nor the lab want it to be obvious that laboratories are being allowed to perform with less QC and higher risk.
The bottom line for labs is that you need to pay attention to your document headings and signatures, address the list of CMS error sources, but you don't need to worry about all of your true risks. The IQCP system, even after years of development, remains an arbitrary and amorphous way to plan quality control. An inspector may discuss or recommend that your laboratory perform a better risk assessment or develop a more comprehensive quality control plan, but your laboratory can't be cited for that and you can be confident you will win any argument about your IQCP.
Because after all, what's worse, poor quality control or a poorly formatted document? a missing risk mitigation or a missing signature?
