Links
Links to Holland 2010
Dr. Westgard gave the Haverkate Lecture at the ECAT participants' meeting in 2010. A summary of his presentation there, plus links to more resources related to his talk.
Links to Holland – Haverkate Lecture Award
December 2010
“As an honour for his unique and important contribution to the field of quality improvement in the medical laboratory, Prof. Dr. J. O. Westgard presented the Haverkate Lecture at the ECAT Participants’ Meeting 2010.”
The ECAT Foundation is a leading provider of External Quality Assessment services in haemostasis and thrombosis for laboratories in Europe and includes 2000 members and participants from 29 countries.
This meeting was held in Leiden, Holland, on November 11-12, 2010. The picture below shows Dr. Havarkate and Dr. Westgard after the lecture presentation. 
The title for the Havarkate Lecture was “Quality Planning - Principles and Practices: A new role for Risk Analysis in developing Analytic QC Plans.” Given the need for harmonization and comparability in laboratory testing, medical laboratories need a comprehensive plan for Analytical Quality Management, along with tools and techniques that provide objective and quantitative assessment of the quality and performance of measurement or examination procedure throughout their life-cycle in the laboratory. Dr. Westgard’s lecture focused on the framework for Analytical Quality Management that has evolved from his work on method validation, statistical QC, design of SQC procedures, as well as broader quality management concepts and principles from TQM and Six Sigma and the emerging influence of Risk Analysis for development of Analytic QC Plans.
Risk analysis provides an approach for customizing a laboratory QC Plan to account for the potential failure modes of a particular analytic system operating in a particular laboratory. ISO 14971 and CLSI EP23 guidelines emphasize the use of risk analysis for medical devices and laboratory QC Plans. The approach is evolving and laboratories will need to learn new tools and techniques, such as Failure Modes and Effects Analysis (FMEA) and the Joint Commission Proactive Risk Reduction methodology. It will be critical for medical laboratories to integrate Risk Analysis into the existing framework for Analytical Quality Management (AQM), which is shown below.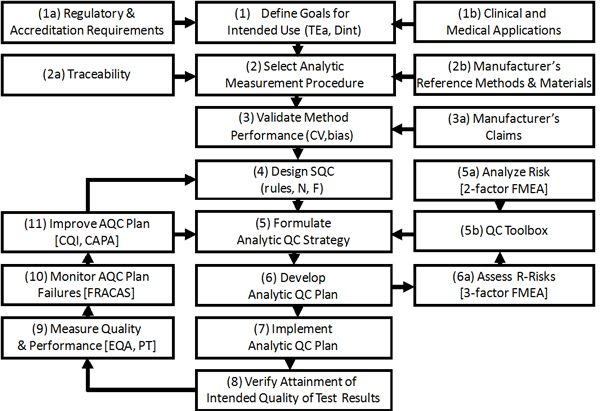
As shown in this AQM framework, the process of managing analytical quality starts with the definition of quality goals for intended use (step 1), selection of analytic measurement procedures (step 2), validation of method performance (step 3), design of Statistical QC (step 4), and formulation of an Analytical QC Strategy (step 5). Six Sigma concepts and metrics are used to define “tolerance limits” in the form of Allowable Total Error requirements, validate method performance by characterizing quality with sigma-metrics, and select appropriate control rules and numbers of control measurements with the aid of a “sigma-metrics QC selection tool.” Sigma-metrics inherently incorporate concepts of risk and defects and therefore can provide guidance for formulating Analytic QC Strategies that relate to risk analysis.
The risk analysis process (Steps 5a, 5b, 6a) should influence the specific details of a QC Plan (step 6) which should always include a properly design Statistical QC procedure. Risk analysis provides the tools to identify potential failures that must be addressed, which influences the specific QC tools (Step 5b) and the formulation of the Analytic QC Strategy (Step 5) and the specifics of the Analytic QC Plan (Step 6). Additional control mechanisms may include operator controls (training, competency, operating checklists), manufacturer’s built-in checks and procedural controls, patient data QC algorithms (delta checks, consistency checks, correlation checks, mean or Average of Normals (AoN), as well as external measures from EQA or PT surveys. The residual risks are evaluated (Step 6a), and if acceptable, the Analytical QC Plan is implemented (Step 7) in order to fulfill the ISO 15189 requirement (Step 8) to verify the attainment of the intended quality of test results. Ongoing monitoring is necessary by EQA, PT, and Peer-Comparison programs, as well as monitoring of specific failure modes (Step 9) within the laboratory, along with efforts for corrective actions, preventive actions, and quality improvement (Steps 10-11). Survey programs, such as provided by ECAT, are critical for the ongoing monitoring of performance and improvement of quality.
If a manufacturer provides the necessary information from its risk analysis studies (Step 5a, 2-factor FMEA), the laboratory’s main effort will be to prioritize failure modes, identify appropriate QC tools (Step 5b), and formulate an Analytic QC Strategy (Step 5). The 1st FMEA will hopefully come from the manufacturer and require little work by the laboratory. However, the 2nd FMEA (Step 6a) is the laboratory’s responsibility and should employ a 3-factor risk model that includes detection, along with the recommended scales for assessing occurrence and severity, estimations of detection for control mechanisms using power curves, then calculation of risk as a defect rate and residual risk as the number of potentially harmful test results that would be produced in a certain period of time.
Westgard Web has been developing educational materials on Risk Analysis to support the emerging ISO/CLSI guidance. Two internet modules are currently available and a third is under development and expected to be posted by mid 2011 (when the CLSI EP23 document becomes final):
1. Introduction – First, do no harm!
2. ISO and CLSI Guidelines
3. Methodology and Tools (coming in 2011)
Meanwhile, here are some materials on this website that should be helpful to prepare for the emerging applications of Risk Analysis in Analytical Quality Management:
- How is Risk Assessed?
- Failure Modes of Risk Assessment
- Risk Analysis Methodology
- The Risk Management Process
- First, Do No Harm
