CLIA Final Rule
Example IQCP for C. Difficile
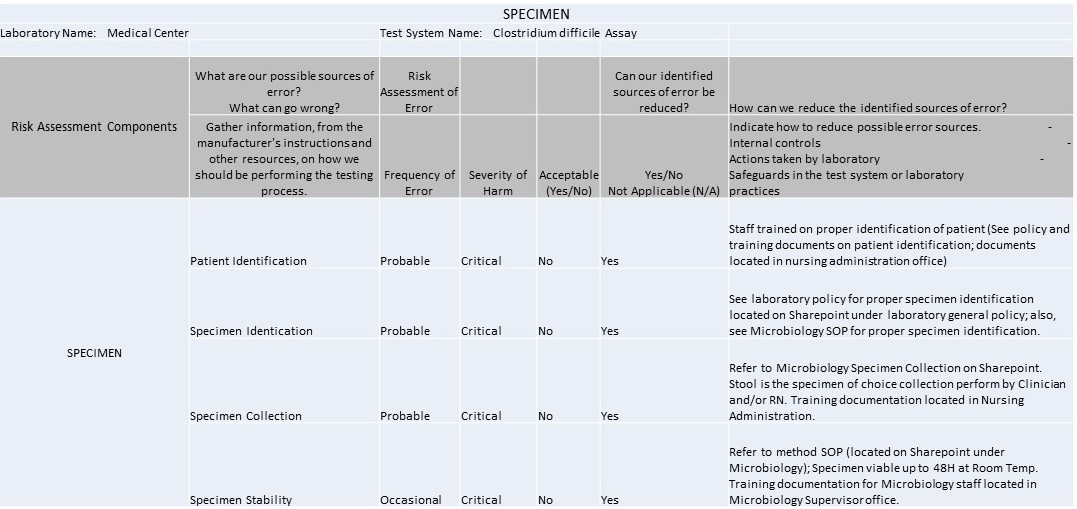
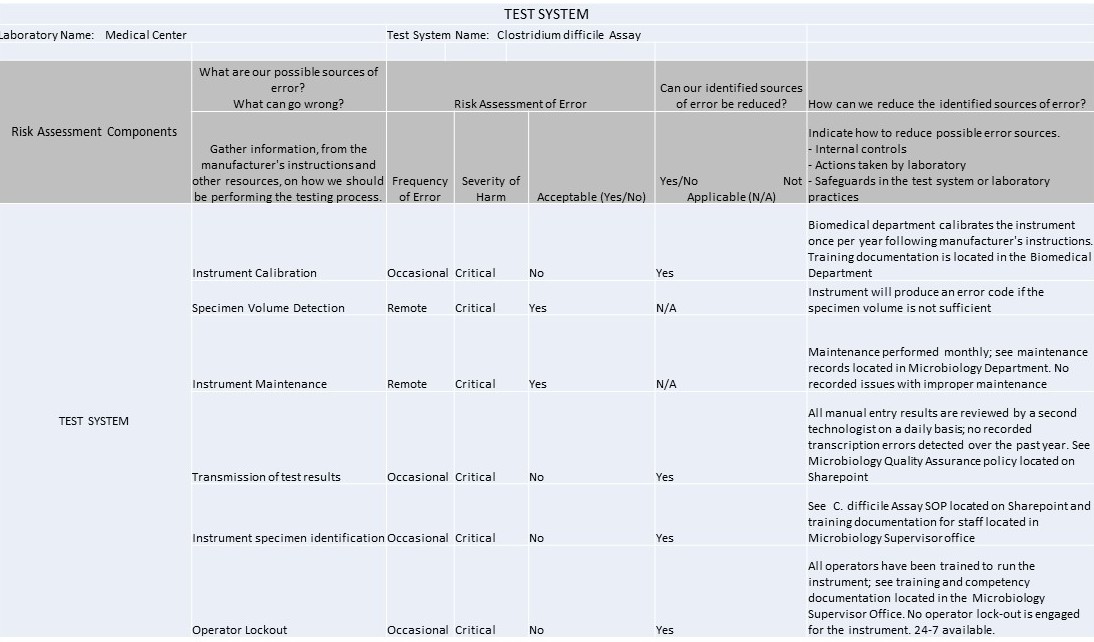
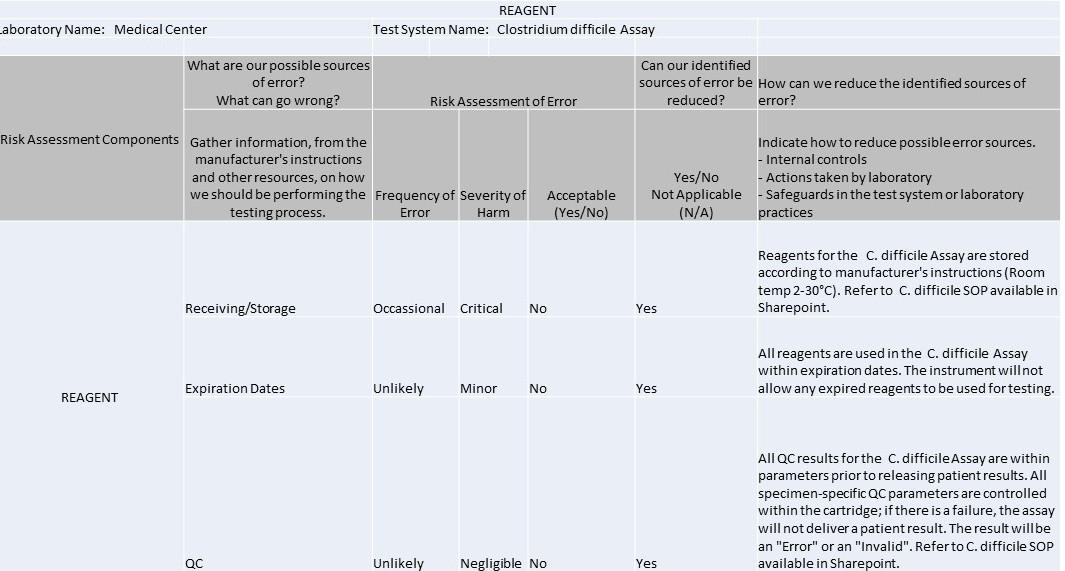
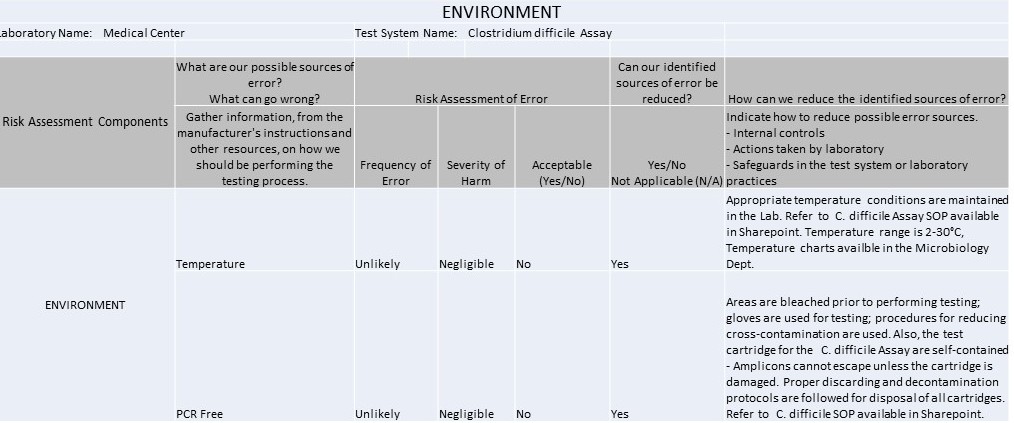
It's 2016 and IQCPs are the law of the (US) land. So what can you make with 1 spreadsheet, 7 worksheets, 37 rows, 42 columns, and 104 completed cells? An IQCP built for a C. Difficile method.
An Example IQCP for a C. Difficile method
Sten Westgard, MS
January 2016
The IQCP regulations have kicked in, and it's time to start sharing practices and templates with each other. We're lucky enough to have one of the Westgard website members willing to share their IQCPs with us. These IQCPs were developed with the help of the CLSI's EP23[trademarked], CMS directions, webinars and information from different vendors and publications. The CMS spreadsheet for IQCP was the source for the worksheets displayed below.
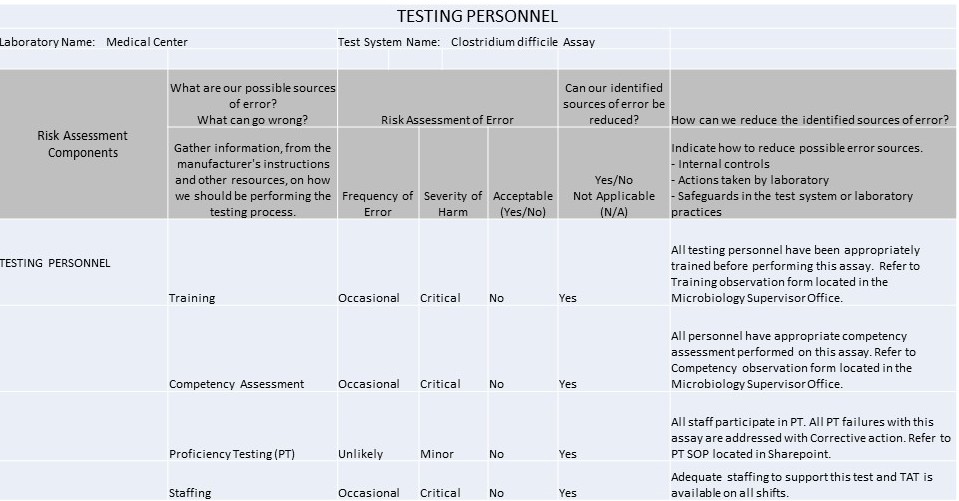
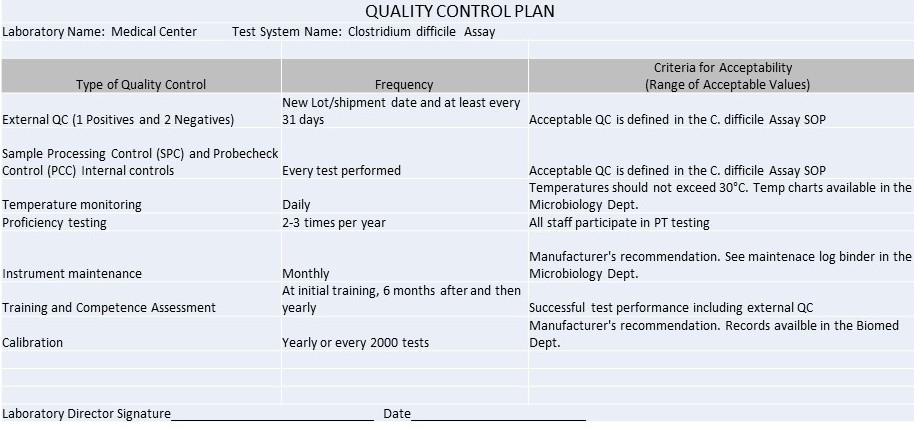
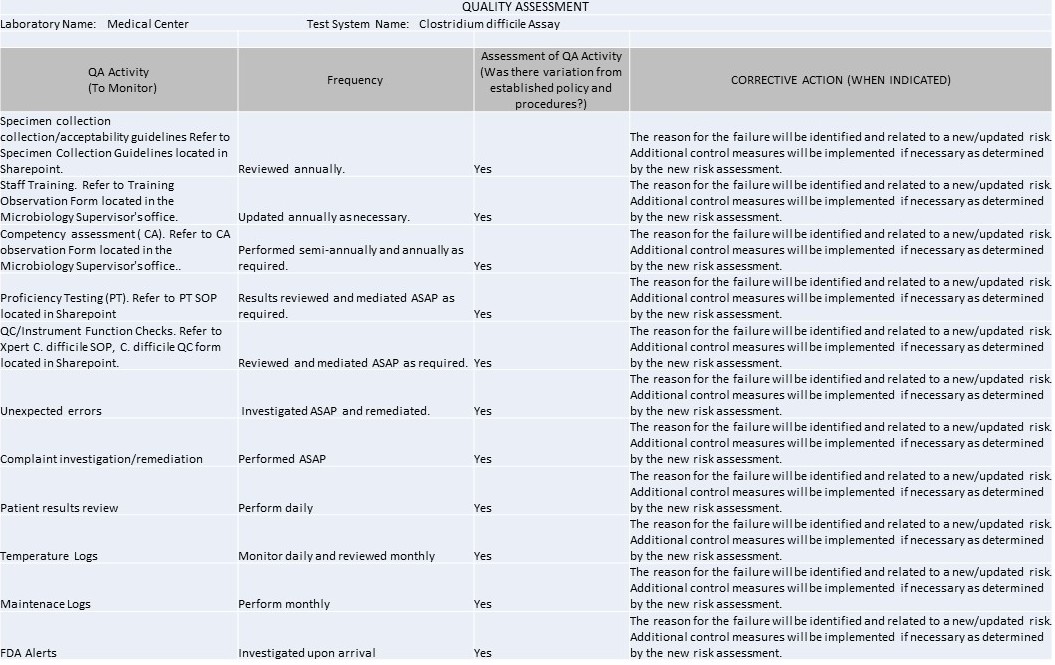
Remember, every IQCP must be individualized by every laboratory. You cannot simply cut and paste the examples below here into your own IQCP, but you can use the same topics, risks, failure modes, etc., and then evaluate them to the particular context of your laboratory.
Specimen
Test System
Reagent
Environment
Testing Personnel
Quality Control Plan
Quality Assessment
This and 4 other IQCPs were prepared by this laboratory. It took 60 to 80 hours of staff work from the laboratory director and the QA coordinator, plus additional work from section supervisors. Each supervisor took 2-4 hours to develop a first draft of their IQCP and then the laboratory director and QA coordinator reviewed and revised the program.
While we here at Westgard QC may disagree with the implementation of IQCP in laboratories and some of the conclusions that labs reach, there are still benefits from sharing the experiences of other laboratories who must implement these regulations.