QC Design
Cholesterol, European Biologic Goal
Recommendations on how to use the QC Validator program with the European biologic goals for imprecision and inaccuracy have been published, along with some example applications [1]. Two additional applications are illustrated here to facilitate comparisons between the European biologic goals, the CLIA analytical quality requirements for total error, and the clinical quality requirements for medically important changes.
Note: at this time this was written, QC Validator was the QC Design software available. Validator has been replaced by EZ Rules 3, which has all the features and capabilities of the earlier version, as well as new enhancements.
Cholesterol:
- 1. Define the Quality Requirement
- 2. Evaluate the analytical factors
- 3. Enter parameters in computer program
- 4. Obtain OPSpecs chart
- 5. Assess probabilities of rejection
- 6. Select the control rules and numbers of control measurements, N
- 7. Adopt a Total QC Strategy
- 8. Reassess for changes in performance
- Conclusions
Cholesterol application
1. Define the quality requirement.
For serum Cholesterol, the EGE-Lab specifications are 4.1 % for bias and 2.7 % for imprecision and therefore, the calculated TEBA is 8.5% (1.65*2.7 % + 4.1% = 8.5 %).
2. Evaluate the analytical factors
For this example, we will take a cholesterol method that demonstrates stable performance of smeas = 2.0 % and biasmeas = 2.0 %; this stable performance is acceptable according to the individual criteria.
3. Enter parameters in computer program
|
To use the QC Validator program to assess the QC necessary to detect unstable performance, parameters can be entered as shown here. Candidate QC procedures are selected manually from the long list ("From Table") to include mean/range procedures which are implemented more often in Europe. The NCEP recommended decision level of 200 mg/dL corresponds to 5.17 mmol/L (commonly used units in Europe). |
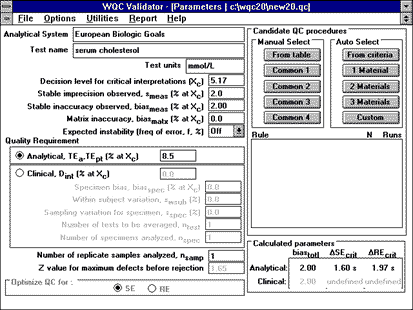 |
4. Obtain OPSpecs chart
| The Option menu is used and the OPSpecs option selected to generate a chart. The OPSpecs chart for a TEa of 8.5% with 90% AQA shows that it will be difficult to control this method and detect unstable performance. The method's operating point (that represents smeas of 2.0% as the x-coordinate and biasmeas of 2.0% as the y-coordinate) is above the operating limits of these single rule, multirule, and mean/range QC procedures all having Ns of 4, which indicates that these candidate QC procedure cannot provide 90% detection of a systematic error that would cause a test result to exceed an 8.5% total error. | 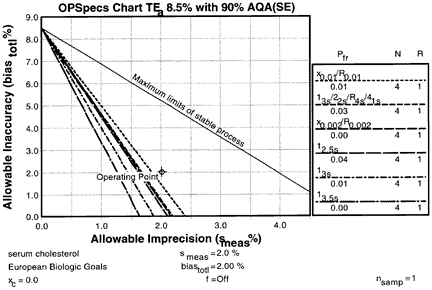 |
5. Assess probabilities of rejection
| Using the Options menu, a critical-error graph can be viewed. This critical-error graph shows that the x0.01/R0.01 rules with N=4 would provide 70% error detection with only a 1-2% false rejection rate. | 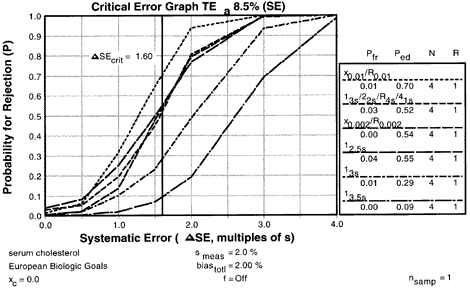 |
6. Select the control rules and numbers of control measurements, N
This x0.01/R0.01 control rule may be an appropriate QC procedure for a method that has excellent stability. When mean/range rules were added to the program's selection criteria, the automatic QC selection function for 3 control materials indicated that the x0.01/R0.01 rules with N=6 would provide 90% AQA.
7. Adopt a Total QC Strategy
|
Since the x0.01/R0.01 rule with N=4 provides 70% error detection (less than 90%), you should use a moderate TQC strategy that balances statistical and non-statistical components. In this table, the relative number of x's indicate the relative emphasis on the different components in a Total QC strategy. SQC stands for Statistical QC. QI stands for Quality Improvement. Other QC includes preventive maintenance, instrument function checks, performance verification tests, and patient data QC. |
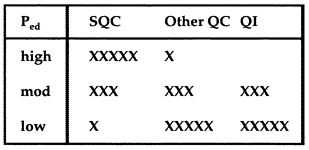 |
8. Reassess for changes in performance
An improvement in performance, i.e. reductions of method bias from 2.0% to 0.0%, would allow this method to be controlled with 90%AQA using x/R or multirule QC procedures with Ns of 4 per run.
Conclusions
The calculated European total error requirement is seen to be more demanding than the NCEP clinical requirement where it was demonstrated that a 12.5s rule with N=2 would provide 90% error detection with only 3% false rejections (see QC planning application for cholesterol with a clinical quality requirement). The European requirement is also more demanding than the US CLIA requirement where it was demonstrated that a multirule procedure with N=4 could provide 90% AQA (see QC planning application for cholesterol with analytical quality requirement). Desirable "purchase specifications" would be a bias of zero and imprecision of 1.9% or better to assure the quality required by the European biologic goals, an imprecision of 2.3% or better for the CLIA quality requirement, and an imprecision of 3.0% or better for the NCEP clinical quality requirement (based on consideration of mean/range, single rule, and multirule QC procedures with N's of 2).
References
- Hyltoft Petersen P, Ricos C, Stockl D, Libeer J-C, Baadenhuijsen H, Fraser CG, Thienpont L. Proposed guidelines for the internal quality control of analytical results in the medical laboratory. Eur J Clin Chem Clin Biochem 1996;34:983-989.
- Westgard JO, Seehafer JJ, Barry PL. European specifications for imprecision and inaccuracy compared with operating specifications that assure the quality required by US CLIA proficiency-testing criteria. Clin Chem 1994;40:1228-1232.
- Westgard JO, Seehafer JJ, Barry PL. Allowable imprecision for laboratory tests based on clinical and analytical test outcome criteria. Clin Chem 1994;40:1909-1914.
- Hyltoft Petersen P, Fraser CG. Setting quality standards in clinical chemistry: Can competing models based on analytical, biological, and clinical outcomes be harmonized? Clin Chem 1994;40:1865-1868.Page references for CLIA QC requirements
