Trends
Who will care for quality tomorrow?
There is a crisis in laboratory staffing; the demand for laboratory professionals has exceeded the supply by thousands. Yet fewer and fewer students are pursuing medical technology. Dr. Westgard examines why this is, what impact it will have on the laboratory, and what solutions will be devised to deal with the scarcity of staffing.
- Who wants to work in a lab?
- What will happen to quality tomorrow?
- What is needed to validate quality?
- What is required for technical validation of quality?
- What progress is being made with technical validation?
- How can technical validation be acheived today?
- How should technical validation be assured tomorrow?
- Conclusion: Who will make it happen?
- References
That's the question on the cover of the August 2000 issue of Medical Laboratory Observer. The featured article [1] discusses the growing shortage of clinical laboratory analysts in U.S. laboratories. Available figures show that there are a total of about 5000 newly trained analysts available each year and that the need is for 9,300 per year through 2008. That's a gap of over 4,000 analysts per year or a total of 40,000 in the next decade. Given there are about 300,000 analysts working in laboratories today, that translates to an expected vacancy rate of over 10%. Many laboratories already are experiencing staffing vacancies that approach 10%, thus the vacancy rates can be expected to approach 20% in the next decade. Laboratories have been doing more with less and will need to do even more with even less in the new millennium.
Diana Mass, writing in the June 2000 issue of the ASCLS Today newsletter, explains the depth of the problem [2]. It's not just low salaries, as suggested by the MLO article, it's that laboratories have become the sweatshops of the new healthcare economy. Healthcare organizations treat laboratories like third-world production factories in order to make money. Laboratorians, being dedicated and conscientious people, work longer and harder to meet the production demands. Productivity continues to improve through the use of more and more automation. However, doing more with less has become a downward spiral, rather than a rewarding journey. If you doubt this, consider whether anyone who works in a laboratory today would advise their own children to follow in their footsteps! What careers are your own children pursuing?
What will happen to quality tomorrow?
Automated analyzers are getting better and better, performing a wider variety of tests faster than ever before. They are also capable of producing more bad results faster than ever before. Everyone assumes that any issues related to quality will be solved by the manufacturers of the analytical systems and by the CLIA regulation of laboratories. However, a recent action by the FDA has shown that manufacturers still have quality problems that need to be solved, thus laboratories can't just buy quality off the shelf [3]. And, the long-awaited final, final, final, final CLIA regulations are expected to further dilute the requirements for quality by eliminating any review and approval of manufacturers QC instructions [4]. In short, laboratories are still responsible for assuring the quality of laboratory tests.
In the past, laboratories depended on highly trained analysts who were skilled in quality control. That solution won't work in the future because laboratories won't have enough analysts with enough time or enough training or enough skills to assure the quality of laboratory test results. In the future, the quality of laboratory tests must be built into automated analyzers, data analysis workstations, and laboratory information systems. The validation of laboratory test results must be completely automated as part of the automation of the laboratory.
What is needed to validate quality?
A complete system for validation of laboratory test results would consist of five components or data "filters", according to Ulenkate, Oosterhuis, Osmanovic, and Goldschmidt [5]:
- Administrative validation monitors the patient identification, specimen type, etc.;
- Technical validation assures the analytical system is performing within the required analytical quality;
- Sample validation considers sample volume, integrity, hemolysis, lipemia, etc.
- Patient validation assesses the consistency of multivariate patient results by a series of correlation rules;
- Clinical validation provides reflex testing, consultative guidance, etc.
All these components are important, however, technical validation is the most basic requirement - if the analyzers don't provide the necessary analytical quality, a laboratory can not to produce valid test results.
What is required for the technical validation of quality?
My view of technical validation is shown in the accompanying figure. Technical validation is more than traditional QC, as currently practiced in U.S. laboratories. Technical validation involves the objective and quantitative management of analytical quality, beginning with definition of the quality required by a test, validation that the analytical method can produce test results of the necessary quality, selection of an appropriate statistical QC procedure on the basis of the quality requirement for the test and the imprecision and inaccuracy observed for the method, collection of control data to monitor the quality of routine runs, and concluding with the release of test results that meet defined requirements for quality.
The analytical quality of laboratory tests can not be objectively managed unless standards or requirements for quality are defined. How good is good enough for a laboratory test? How close does the reported value have to be to the correct answer? The lack of defined requirements for quality (step 1) and the lack of a quality-planning process (step 3) are the main limitations in current quality systems for technical validation.
What progress is being made with technical validation?
Recently, a consensus was achieved on how to define quality requirements for laboratory tests [6]. A hierarchy of quality standards was accepted that includes several different types of requirements that embody different concepts and require different formats:
- Biologic goals are defined by a maximum allowable CV (sbmax) that describes only the imprecision of the analytical method and a maximum allowable bias that describes only the inaccuracy of the analytical method (biasbmax).
- Allowable total error (TEa) encompasses the imprecision and inaccuracy of the analytical method;
- Medically important change or clinical decision interval (Dint) encompasses both analytical and pre-analytical factors that affect the variability of tests;
Sources of information on each are available in the literature, such as the European recommendations for biologic goals [7] and a large data-base of information about biologic variability and desirable specifications for bias, CV, and total error [8]; allowable total errors as defined by the US CLIA acceptability criteria for proficiency testing [9] or external quality assessment programs in other countries; and clinical decision intervals as available from test interpretation guidelines such as the US National Cholesterol Education Program [10] and from Skendzel and Barnett's table of medically important changes in test results [11].
There also has been progress in defining a quality-planning process to select appropriate statistical QC procedures. The recent CLSI document C24-A3 describes principles for applying statistical QC procedures with quantitative laboratory measurements [12] and includes a section on "planning a statistical quality control procedure." A practical quality-planning process can be implemented with the aid of a graphical tool - the chart of operating specifications (or OPSpecs chart) - that shows the relationship between a defined quality requirement and the imprecision and inaccuracy that are allowable for different controls rules and numbers of control measurements [13,14].
It should now be possible to objectively and quantitatively manage the analytical quality of laboratory tests. What remains is to provide the technology that makes technical validation easy and practical in today's busy laboratories. That means developing the computer technology and incorporating these capabilities in the software of instruments, data workstations, or laboratory information systems.
How can technical validation be achieved today?
That's the ultimate objective of the QC Validator technology that we're developing. The heart of the technology is the methodology for selecting statistical control rules and numbers of control measurements on the basis of the quality required for a test, the imprecision and inaccuracy observed for a method, and the rejection characteristics of different statistical decision criteria. See the discussion of the EZ Rules program on this website to learn how you can select valid QC procedures today! You can begin by following these simple steps:
- Define the quality required for your tests using the CLIA proficiency testing criteria.
- Estimate the precision of your methods from current QC data and estimate the bias from recent proficiency testing data; you can even assume bias is zero as a starting point.
- Select appropriate statistical control rules and numbers of control measurements using our OPSpecs methodology, as available with Internet tools on this website, manual tools in our Basic Planning for Quality book, or computer tools in our EZ Rules 3 program.
- Implement the appropriate statistical QC procedure with your analyzer's QC software, your PC QC workstation, your laboratory information system, or manually if necessary.
- Perform QC following good laboratory practices, such as outlined in our Basic QC Practices manual.
How should technical validation be assured tomorrow?
Validator3 - our most advanced QC design program - can be used to prepare an automatic rule selection engine that can be embedded in the software of an analytical instrument, PC data work station, or laboratory information system. As can be seen from the QC Instructions form, all the critical input parameters can be provided in a single computer screen to support the automatic selection of statistical QC procedures. Even some of these inputs might be eliminated by having the the analyzer provide ongoing estimates of the method's imprecision and inaccuracy. The user might select sources of information for the estimates of imprecision (e.g., replication experiment or current QC data) and inaccuracy (e.g., comparison of methods experiment or current proficiency testing or external quality assessment data), thereby providing input parameters that will reflect the up-to-date performance of the analytical method.
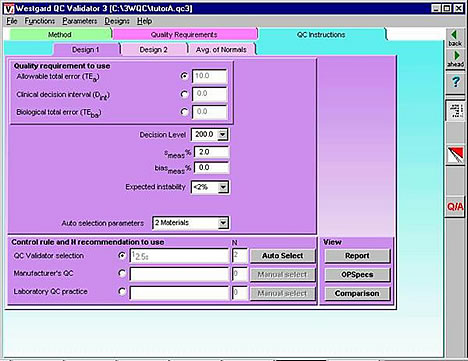
By adding a feedback loop and by making the automatic design "live," the QC process itself could be completely automatic and dynamic, i.e., the QC procedure could automatically change if the method performance characteristics changed. An improvement or reduction in a method's CV would automatically lead to fewer or less complex control rules and fewer control measurements. A deterioration in method performance would automatically cause the QC system to increase the number of control measurements or change the control rules, first narrowing the limits on single rule procedures then adding rules to increase error detection via multirule procedures. A dynamic QC process would account for performance observed in an individual laboratory and the quality required by the patients served by that laboratory. The quality of test results could be guaranteed even when there are differences in requirements for different medical services and differences in performance from laboratory to laboratory. That's what must be achieved to assure the technical quality of laboratory test results in the future.
Conclusion: Who will make it happen?
The WHO is us. The TIME is now. We must make the improvements that are needed for the future today!
In the future, no one will know what needs to be done because the technical knowledge and skills will have been lost. There won't be any quality management, just compliance management! The only measure of performance of interest to management will be dollars! The future is now and we have a chance to change it!
Manufacturers will implement improved quality management techniques and technology IF customers demand improvements. In the absence of a strong voice from the customers, nothing will change. We must demand improvements in QC technology when we purchase new instruments and information systems. We must express the needs for better QC technology when we participate in marketing surveys and focus groups. We must include statements of quality requirements when we write specifications to purchase new analytical methods. We must complain to manufacturers when their products do not meet our requirements for quality.
We must make the future happen in our lifetimes. "We must care for quality today if we are to be recipients of quality care tomorrow." [15]
References
- Kipp J. Who wants to work in a lab? MLO 2000;Aug:25-29.
- Mass D. Clinical Laboratory Sciences: Victims of our own success. ASCLS Today newsletter. 2000;XIV(No 6, June):5-6.
- Westgard JO. A wake-up call for laboratory quality management.
- Westgard JO. Sage advice about new approaches to quality control.
- Ulenkate HJLM, Oosterhuis WP, Osmanovic N, Goldschmit HMJ. Selfreporting validation software ('VALAV' and 'LabRespond') compared with clinical chemists. Private correspondence with
This email address is being protected from spambots. You need JavaScript enabled to view it. - Kenny D, Fraser CG, Hyltoft Petersen P, Kallner A. Consensus agreement: Strategies to set global analytical quality specifications in laboratory medicine. Scand J Clin Lab Invest 1999;59:585-586.
- Frazer CGF, Hyltoft Petersen P, Ricos C, Haeckel R. Proposed quality specifications for imprecision and inaccuracy of analytical systems for clinical chemistry. Eur J Clin Chem Clin Biochem 1992;30:311-317.
- Ricos C, Alverez V, Caba F, Garcia-Lario JV, Hernandez A, Jiminez CV, Minchinela J, Perich C, Simon M. Current databases on biologic variation: pros, cons and progress. Scand J Clin Lab Invest 1999;59:491-500.
- U.S. Dept. of Health and Social Services. Medicare, Medicaid, and CLIA Programs: regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA). Final Rule. Fed Regist 1992;57:7002-7186 (February 28 1992 issue).
- National Cholesterol Education Program Laboratory Standardization Panel. Current status of blood cholesterol measurements in clinical laboratories in the United States. Clin Chem 1988;34:193-201.
- Skendzel LP, Barnett RN, Platt R. Medically useful criteria for analytic performance of laboratory tests. Am J Clin Pathol 1985;83:200-205.
- NCCLS C24-A2. Statistical quality control for quantitative measurements: Principles and definitions: Approved guideline - 2nd edition. National Committee for Clinical Laboratory Standards, Wayne, PA, 1999.
- Westgard JO. Error budgets for quality management: Practical tools for planning and assuring the analytical quality of laboratory testing processes. Clin Lab Manag Review 1996;10:377-403.
- Westgard JO. Basic Planning for Quality. Madison, WI:Westgard QC, Inc., 2000.
- Westgard JO, Burnett RW, Bowers GN. Quality management science in
clinical chemistry: a dynamic framework for continuous improvement of
quality. Clin Chem 1990;36:1712-1716.
James O. Westgard, PhD, is a professor of pathology and laboratory medicine at the University of Wisconsin Medical School, Madison. He also is president of Westgard QC, Inc., (Madison, Wis.) which provides tools, technology, and training for laboratory quality management.
