Sigma Metric Analysis
Sysmex XR, manufacturer's study, multimode analysis
In 2021, Sysmex introduced the next evolution of their XN line, the XR. When the manufacturer runs the study, does it stack up better against the latest CLIA goals, EFLM desirable and minimum goals?
Sysmex XR, manufacturer study, multimode analysis
Sten Westgard, MS
April 2024
Other articles in the multimode series:
- Beckman Coulter DxC 700
- Abbott Alinity
- Abbott Alinity in Turkey
- Abbott HbA1c in USA
- Siemens Atellica
- Siemens Atellica in Romania
- Siemens Atellica in Spain
- Siemens ADVIA 2120i
- Siemens Hema 580 in South Africa
- Roche c501 in Turkey
- Roche c501 in Saudi Arabia
- MicroLab RX-50 in India
- Roche cobas 6000 immunoassays in Turkey
- Sysmex XN 350 in India
- Dymind DH 76 in Bulgaria
- Mindray 7500 in China
- Mindray BS 2000M in China
Sigma Score 2 out of 6. Unhelpful/Optimistic [The only positives of this study are the use of patient samples for comparison purposes, and the application of EFLM goals in the assessment, and the use of a large number of patient samples in the comparison.]
This study is truly international. The imprecision study was conducted in Japan, while the comparison of methods study was conducted in Germany. As is common with hematology instruments, manufacturer controls are used to estimate imprecision.
Performance evaluation of the new Sysmex XR-Series haematology analyser. Fujimaki K, Hummel K, Magonde I, Dammert K, Hamaguchi Y, Mintzas K, Saker J, Valina O, Otte KM. Practical Laboratory Medicine 39 (2024), e00370. https://doi.org/10.1016/j.plabm.2024.e00370
The Imprecision and Bias data
"The analytical performance study was conducted in Sysmex R&D centre (Kobe, Japan) with the use of control blood XN CHECK Level 1/Level 2/ Level 3 or fresh peripheral blood samples from volunteers...The precision study was conducted... in accordance with the CLSI EP05-A3 method. Two replicate measurements of XN CHECK Level 1/ LEvel 2/ Level 3 were performance in 2 runs (morning and afternoon) for 20 test days. The two-way nested ANOVA test was used to analyse the 80 measurements obtained. Variations within the day, bewteen day and overall, within-laboratory precision were calculated....The reproducibility study was conducted...in accordance with the CLSI EP05-A3 method. Five replicate measurements of XN Check Level 1/Level 2/Level 3 were performed on three XR analysers for five consecutive days. The two-way nested ANOVA test was used to analyse the 75 measurements obtained." For the imprecision used in this assessment, we took the within-laboratory estimate."
"7,460 venous blood samples from the routine haematology laboratory (Medilys) at Asklepios Clinic Alton in Hamburg, Germany were collected in K2EDTA anticoagulated tubes....The study cohort included all samples received by the laboratory before 12 a.m., and all samples received after 12 a.m. with a request for smear review, for a period of one working week....Correlation coefficients and regression equations were calcualted for all parameters...."
We used the regression equation to estimate bias at the levels where the Sysmex controls were run.
| Sysmex XR CLIA 2024 | ||||
| Analyte | Level | % Bias | %CV | Sigma |
| Hemoglobin Level 1 | 57.4 | 0.619 | 0.91 | 3.7 |
| Level 2 | 114.3 | 0.709 | 0.59 | 5.6 |
| Level 3 | 154 | 0.732 | 0.67 | 4.9 |
| Hematocrit Level 1 | 17.37 | 0.295 | 1.17 | 3.2 |
| Level 2 | 34.34 | 0.841 | 1.09 | 2.9 |
| Level 3 | 44.85 | 0.972 | 1.16 | 2.6 |
| Platelets Level 1 | 105.9 | 0.577 | 5.71 | 4.3 |
| Level 2 | 263.2 | 0.710 | 3.34 | 7.3 |
| Level 3 | 553.1 | 0.757 | 2.45 | 9.9 |
| RBC Level 1 | 2.34 | 0.238 | 1.00 | 3.8 |
| Level 2 | 4.365 | 0.475 | 1.08 | 3.3 |
| Level 3 | 5.22 | 0.610 | 1.08 | 3.1 |
| WBC Level 1 | 2.83 | 1.825 | 1.98 | 4.1 |
| Level 2 | 6.52 | 1.245 | 1.10 | 8.0 |
| Level 3 | 14.87 | 0.995 | 1.06 | 8.5 |
Sigma-metrics according to EFLM-derived DESIRABLE performance specifications
As set forth by the Milan Heirarchy, performance goals based on biological variation are considered superior to those set by regulators and proficiency testing or external quality assurance programs. However, the hematology goals are known to be very demanding.
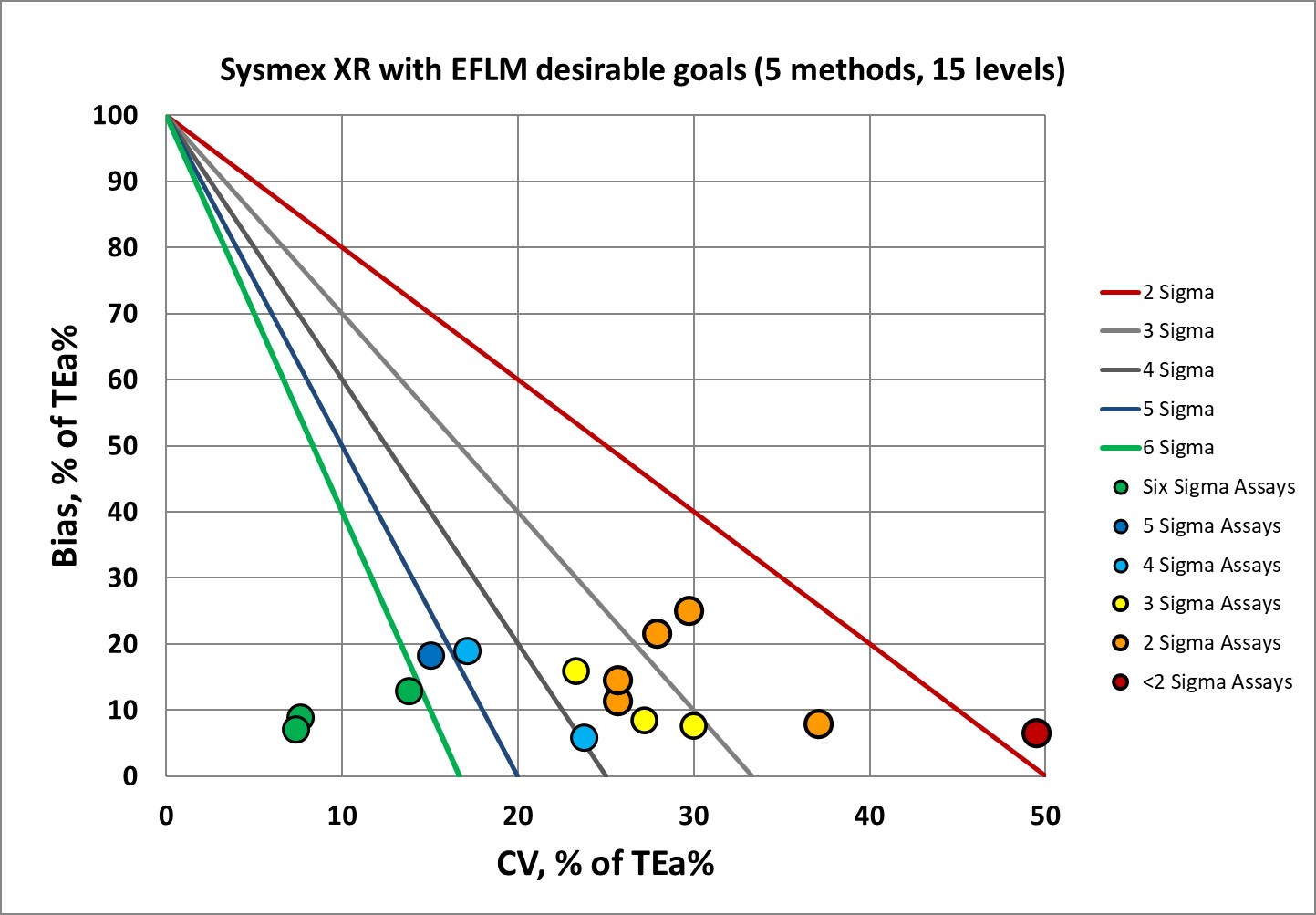
As hematology instruments go, this is pretty good for the desirable specifications. But you can understand why EFLM no longer recommends using desirable performance specifications. Instead, they recommend the minimum performance specifications. So let's see that.
Sigma-metrics according to EFLM-derived MINIMUM performance specifications
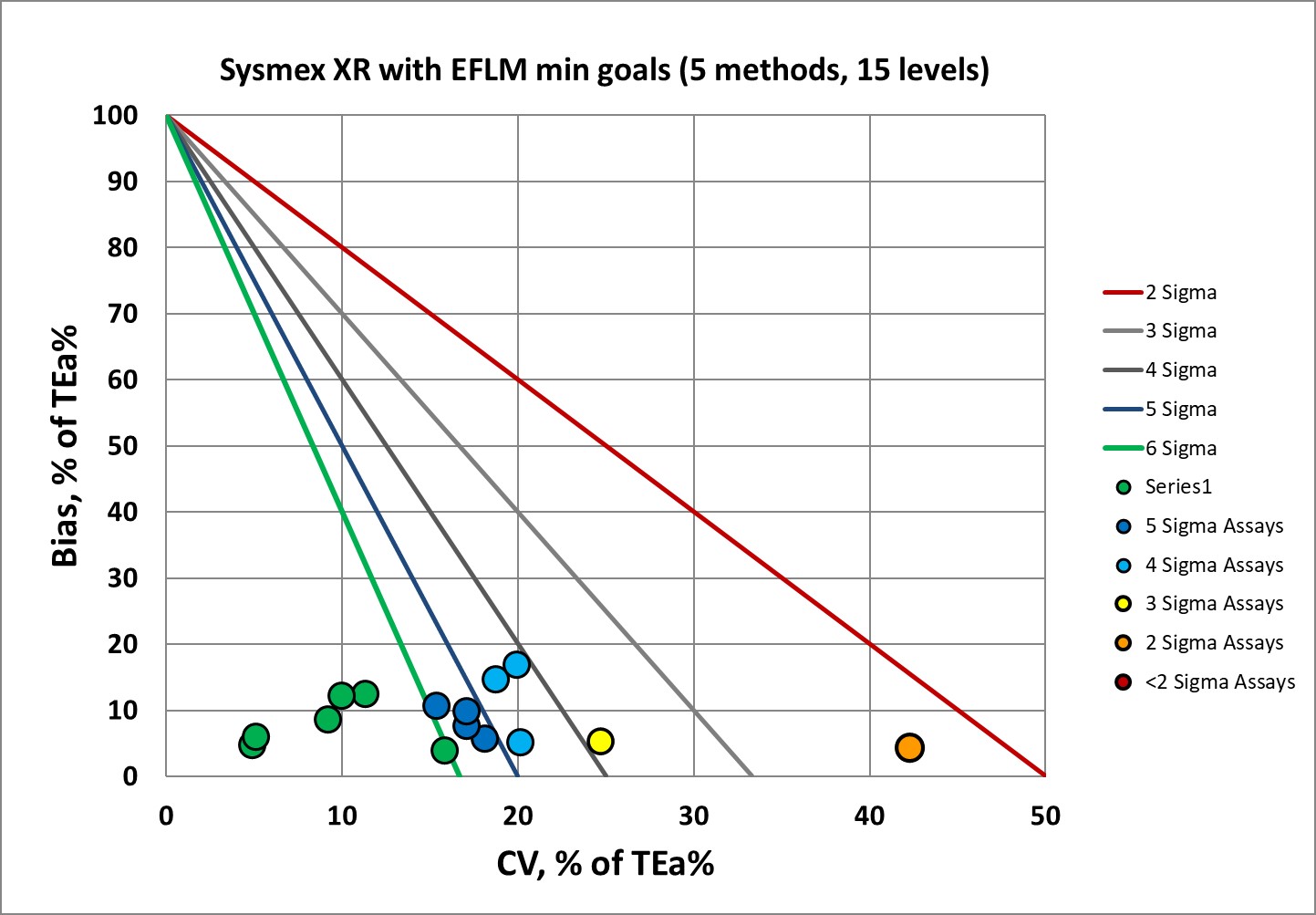
This is quite a nice picture. Maybe not as ideal as one might like, but if the minimum goals are the new benchmark, the XR is doing just fine.
Sigma-metrics according to CLIA 2024 performance specifications
For US labs and labs that are governed by CAP accreditation, there's a respite: the EFLM biological rules aren't mandatory. CLIA 2024 goals are instead. One shortcoming is that the CLIA goals don't cover all the differential parameters, they only cover a few analytes. The 1992 CLIA goals were fairly forgiving, but there's a rude wake up for labs once the new goals kick in.
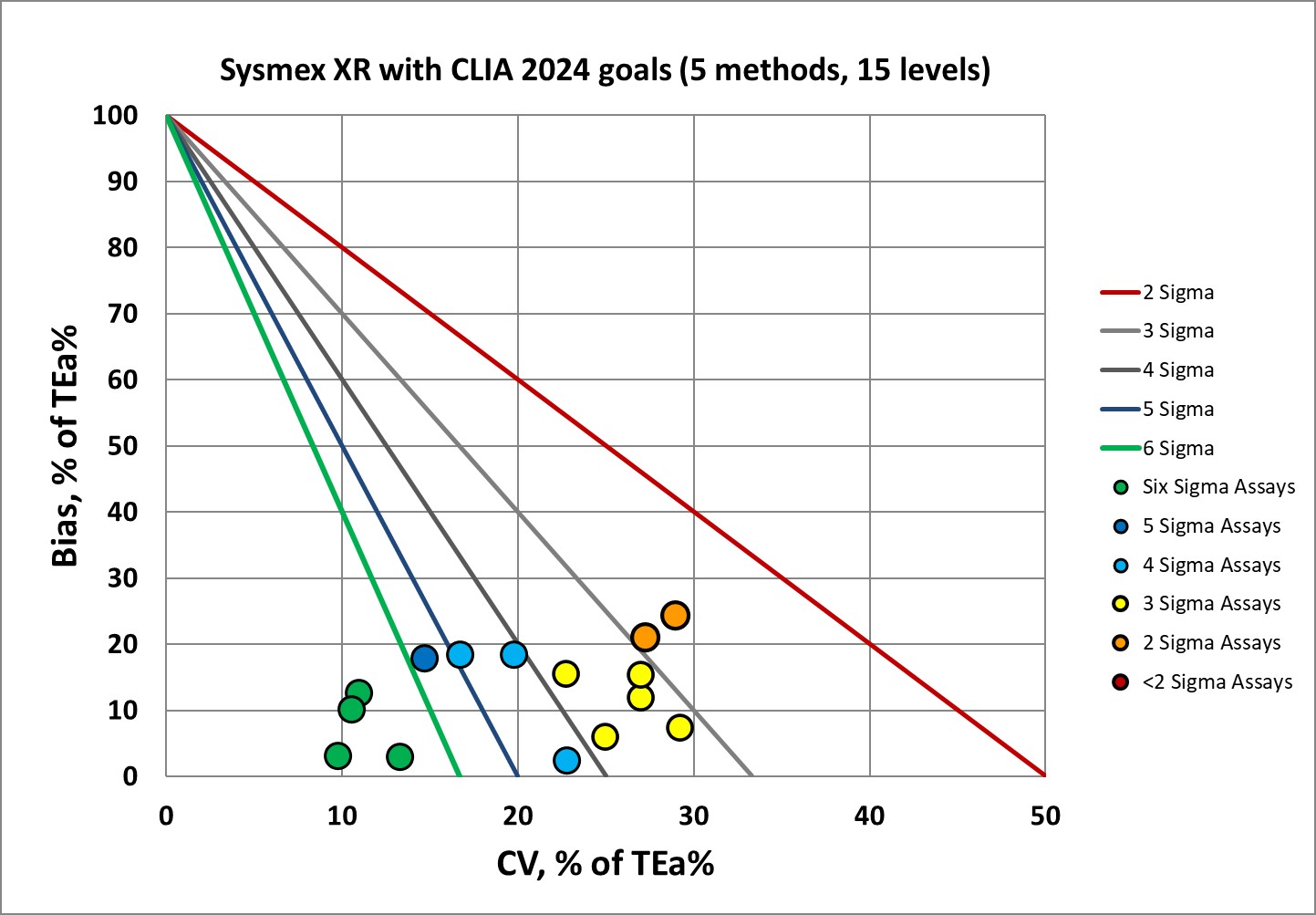
Here's a more mixed picture. There's good news, and there's not all-that-bad news. The platelet method can hit the bull's-eye, but that's because the goal still hearkens back to 1992. CLIA is harder to achieve than the EFLM minimum, so for US labs, there's a harder road to travel.
Conclusion
The authors end the paper with this assessment: "In conclusion, this first XR analyser study showed very good analytical performance for the new XR analyser and in all investigated parameters, flags and workflow aspects, with highly comparable results to the current XN state-of-the-art routine haematology analyser."
If this is the first look at the XR, there is a need to get an independent look at instrument performance. A study not conducted by the manufacturer, but by a customer.
