Risk Management Essays
Identifying Failure Modes
An essential part of Risk Analysis is the identification of what might go wrong - in others words, the failure modes of a process. After diagramming a process, each step of the process must be examined to determine the failure modes. Typically this is done by team brainstorming to identify the potential failure modes, then a summary of those modes through a cause-and-effect diagram, sometimes called a "fishbone" or Ishikawa diagram. (Preview)
Identifying Failure Modes
September 2011
James O. Westgard, PhD
- Patient Testing Process
- Analytic Testing Process
- Basics of Brainstorming
- Potential Failure Modes causing a delay in reporting laboratory test results
- Cause-and-effect or "Fishbone" Diagrams
- What to do?
- References
In any risk analysis methodology, the process of interest must first be diagrammed to identify the critical steps or operations. The next step in the methodology is to identify potential failure modes, i.e., what might go wrong and cause a delay or an error in reporting a test result. The identification of failure modes often makes use of brainstorming, followed by a graphical summary by “fishbone” diagram. Later chapters will consider the prioritization of failure modes to assess their relative importance and guide efforts to reduce the risks of failures, the identification of root causes of the high priority failure modes, and the utilization of risk control option analysis (ISO/CLSI terminology) [1-4] or process redesign strategies (JC terminology) [5] to eliminate causes when possible, detect failures and implement corrective actions for recovery, and
reduce the risk of patient harm by providing information for the safe use of test results.
Patient Testing Process
The overall patient testing process involves many steps and can be extremely complicated. To simplify any risk analysis project, it will be critical to confine the project to a part of the patient testing process, otherwise the project may become unmanageable (i.e. the scope will be so large as to be overwhelmed by a multitude of failure modes).
An overview of the patient testing process is shown below which outlines two major processes – the clinical testing process and the laboratory testing process, the latter often called the “total testing process” by the personnel in a medical laboratory. This laboratory process consists of the pre-analytic, analytic, and postanalytic phases of the patient testing process. It typically begins with the receipt of an order for a test and ends with the report of results. This is the way personnel outside the laboratory usually see the laboratory – a department or “black box” with an input for test requisitions and an output for test results. Their focus is on the “clinical testing process,” or the pre-pre-analytic and post-postanalytic phases of the patient testing process, though they won’t necessarily describe it using this terminology.
In applying risk analysis in a medical laboratory, it will be critical to delineate the scope of the project, particularly whether it crosses the departmental boundaries between the clinical and laboratory processes. Across-department projects will require a more diverse and larger team. For example, projects on patient identification and test turnaround time are inherently difficult because of the number of people and departments that are involved. Even within the laboratory, it is necessary to carefully delineate the start and stop steps to narrow the focus when possible to a primary phase.
For example, when the purpose of the risk analysis project is to develop an Analytic QC Plan, the focus will be primarily on the analytic phase of the laboratory testing process. Still, there could be overlap with the pre-analytic phase related to sample quality and overlap with the post-analytic phase related to implementation of control mechanisms (e.g., delta checks), review of test reports, and provision of information for safe use via the LIS or HIS. The project team may need some representation from different areas in the laboratory department.
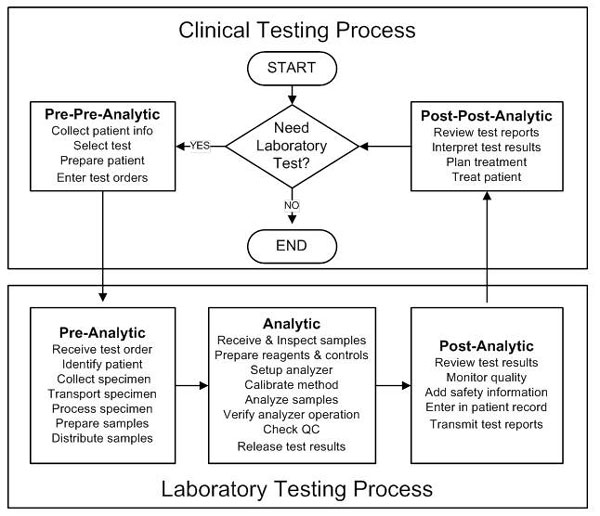
Diagram of the patient testing process, showing the clinical testing process (composed of the pre-pre-analytic and post-post-analytic phases) and the laboratory testing process (composed of the pre-analytic, analytic, and post-analytic phases).
This “big picture” of the patient testing process should provide the frame of reference for narrowing the focus of any risk analysis project. Next, a more detailed description in the form of a flowchart is needed of the specific process of interest. We next focus on the analytic phase of the patient testing process and the identification of potential failure modes that lead to delayed test results and delayed patient diagnosis or treatment.
We invite you to read the rest of this article
 The Six Sigma Risk Analysis manual contains a complete chapter on Identifying Failure Modes, plus 17 more chapters on Risk Analysis, FMEA, ISO standards, and CLSI guidelines related to risk.
The Six Sigma Risk Analysis manual contains a complete chapter on Identifying Failure Modes, plus 17 more chapters on Risk Analysis, FMEA, ISO standards, and CLSI guidelines related to risk.
The rest of this article is not available online - it is only available in the Six Sigma Risk Analysis manual.
References
- ISO 14971. Medical devices – Application or risk management to medical
devices. ISO, Geneva, 2007. - ISO/TC 22367. Medical laboratories – Reduction of error through risk
management and continual improvement. ISO, Geneva, 2008. - CLSI EP18A2. Risk Management Techniques to Identify and Control
Laboratory Error Sources. Clinical Laboratory Standards Institute, Wayne,
PA, 2009. - CLSI EP23P. Laboratory Quality Control Based on Risk Management.
Clinical Laboratory Standards Institute, Wayne, PA, 2010. - Joint Commission. Failure Mode and Effects Analysis in Health Care:
Proactive Risk Reduction. The Joint Commission, Oakbrook Terrance,
IL, 2010.
