Interviews
Sunway faces down the virus in Malaysia
The global pandemic has certainly hit some countries harder than others. Sunway Medical Centre, in Selangor, Malaysia, gives us a picture of what a successful response looks like.
Staring down the Sun: Facing the Pandemic in Malaysia.
April 2019

Dr Jamuna Jairaman

Dr Tang Wai Ying

Dr Norziha Zainul Abidin
Sunway Medical Centre (SUNMED) department of pathology & laboratory medicine commence operations in November 1999. Sunway Medical Centre laboratory is Malaysia’s first private hospital laboratory to be awarded with MS ISO 15189 accreditation standard. We have been sustaining the accreditation for the past 16 years for the following scope of testing; Chemical Pathology, Histopathology, Hematology (inclusive of Transfusion Medicine), Microbiology (inclusive of Immunology Serology) and Cytopathology under the revised version of MS ISO 15189:2014. The Sunway Medical Centre laboratory is the first private medical laboratory in Malaysia to receive the Westgard Sigma Verification in year 2014 and sustaining the certification for the past 7 years. The laboratory services were expanded with two other departments in May 2017 and May 2018, they are the Molecular Diagnostic and Genetic Laboratory and Special Haematology (Stem Cell) Laboratory.
The Department of Pathology and Laboratory Medicine, Molecular Diagnostic and Genetic Laboratory and Special Haematology (Stem Cell) Laboratory offers a range of laboratory services covering both routine and specialist testing. Our extensive testing procedures are performed to help physicians diagnose disease and monitor patient care. The Laboratory services is equipped with technologically advanced instrumentation such Next gene sequencer, Maldi –Tof (VITEK MS), Blood Specimen management system etc. and led by competent laboratory personnel. We also have a team of Consultants to facilitate and sustain the outstanding quality of SUNMED Lab.
Our department of Pathology and Laboratory Medicine runs 3,000,000 tests/ annum, our Molecular Diagnostic and Genetic Laboratory about 120,000 tests/annum, and Special Haematology Laboratory runs around 1,000 tests/annum.
As one of the leading private tertiary medical centres in the country, Sunway Medical Centre is posed to set new standards of service for its growing clientele with a total of 617 licensed beds, 180 consultation suites and 12 operating theatres.
We established a COVID 19 Taskforce Committee on 3rd Feb 2020, involving the relevant stakeholders from the Allied Health Services, Support Services and Clinical Services, the chairman of this Taskforce is the Medical Director, guided by the Infectious Disease Specialist, Physician, Managing Director, Directors and some related Assistant Directors. The first meeting with State Health Department and District Health Office with the taskforce committee members were held on 4th of February.
The head of laboratory was urged to expand COVID-19 testing at Molecular Diagnostic & Genetic Laboratory. The initial discussion on the startup of the Covid-19 lab took place in the taskforce meeting number 5, held somewhere around 7th of February 2020, The laboratory head, was given a month to start this service in-house for Covid-19 RT PCR testing. The lab was established with the help of the scientists at Molecular Diagnostic and Genetic Laboratory, the required space, equipment and resources were planned and procured. As the service started, we had a capacity of 160 tests/ day but this ramped up to 500tests/day and subsequently 1000 tests/day. We had to look at the resources available at that point of time, in terms of man power, equipment for both extraction and PCR, supply, and space, and determine how best to scale them up to meet the need that exists
The kits to be used for the testing was discussed in the taskforce based on the recommendation from IMR (Malaysian Institute of Medical Research). Two of our scientist were sent for a briefing session on the Laboratory testing protocol for Covid-19 on 6th of February 2020, in this briefing there were sharing session from the Head of Virology Unit and Molecular Virologist on the testing algorithm, target genes to be included, WHO and CDC guidelines, MOH protocol, the available recommended kits, specimen type and the quality control testing. All testing Laboratory in Malaysia has to be approved by MOH prior to offering the molecular testing for Covid-19. We were given positive control of inactivated Covid -19 to perform real time RT-PCR using the commercial kits that our laboratory intends to use at SunMed facility and we are required to send the images of the PCR amplification curves to the Head of Virology Unit and Molecular Virologist. We were advised, as with WHO guidelines for testing of COVID 19, the targets for the real time RT-PCR for Covid 19 must include both "E" gene and "RdRp" gene. We obtained the approval from National Public Health Laboratory and IMR to be listed as one of the MOH listed laboratory for Covid 19 testing. On 24th of February, 2020 the test was offered catering only for inpatients with a turnaround time of 1 day. All positive results were required to send to IMR for verification purpose.
Sunway Medical Centre implemented multiple methods for testing:
|
Test |
Kits/Method |
Turnaround time |
|
Covid 19 RT PCR |
Assay: Allplex 2019-nCoV Assay |
10 to 12 hours |
|
Covid 19 Antigen |
Manufacturer: SD Biosensor |
1 hour |
|
Covid 19 Antibody - IgM and IgG |
Manufacturer: SD Biosensor |
1 hour |
|
Covid 19 - Rapid Molecular testing |
Manufacturer: ID Now, Abbott POCT |
2 hours |
The guidelines and availability of test methods and test results evolved rapidly:
|
Duration |
Cut-off time for COVID-19 PCR test sample processing |
Runs /day |
Operation hours |
Manpower |
Average samples per day |
Average samples per run ranges from |
|
Feb 2020 to April 2020 |
10:00 AM |
2 |
1 SHIFT = coverage for 8 hours · Overtime paid for extra hours |
2 to 4 |
85 |
35 to 96/ run /equipment |
|
May 2020 to Aug 2020 |
12:00 PM |
4 |
2 SHIFTS = coverage for 16 hours · Overtime paid for extra hours |
6 to 8 |
400 |
|
|
5:00 PM |
||||||
|
10:00 PM |
||||||
|
4:00 AM |
||||||
|
Sept 2020 to Jan 2021 |
8:30 AM |
7 |
3 SHIFTS= coverage for 24 hours |
10 to 12 |
Ranging from 500 to 1200 |
|
|
1:00 PM |
||||||
|
3:00 PM |
||||||
|
6:00 PM |
||||||
|
10:00 PM |
||||||
|
4:00 AM |
||||||
|
Feb 2021 to March 2021 |
12:00 PM |
4 |
3 SHIFTS= coverage for 24 hours |
6 to 8 |
Ranging from 300 to 400 |
|
|
5:00 PM |
||||||
|
10:00 PM |
||||||
|
4:00 AM |
Verification and Validation of Methods
In the initial stage we verified methods by correlating our results with IMR, approximately 10 positive samples were verified against IMR Lab.
For all new platforms, before proceeding on offering the test, a verification study is done on the archived known positive and negative clinical samples (tested before on other platform). The outcome showed PPA (positive percent agreement) and NPA (Negative percent agreement) of ≥ 97%.
We participate in the INTERLABORATORY COMPARISON PROGRAMME FOR COVID 19 rt PCR TESTING offered by the National Public Health Laboratory. The results showed 100% concordant between our result and the provider’s result. EQA Molecular SARS-CoV-2 was performed on Allplex 2019-nCoV assay and the results showed 100% concordant.
Currently, we run Manufacturer in-kit (Positive control, negative control and internal control). These controls are done on every run. Quality controls are measured by performing the internal, negative and positive controls, all the control readings have to be valid for the results to be released. Most of the times the QC turns out to be valid and passed. Occasionally, you will get invalid or failed control readings. When either of the control showed invalid or internal control detected in negative control would be a possible cause of cross contamination that occurred during manual handling of samples for rT PCR tests. The test will be repeated.
Manufacturer QC is considered reliable as the assay had undergone analytical performance evaluation shown with statistical data shown in their package insert for determination of specificity, sensitivity and limit of detection of the assay. It works best for the diagnostic laboratory who needs to run the test on timely manner. However, it is advisable to use independent, third party quality control. Not only are there advantages for efficiency by being able to be used across multiple platforms, they also react as an unbiased, independent performance check.
We ensure 3 to 6 months’ stock of reagent, swabs and viral transport medium for PCR. As we were worried on delay in shipment as well as shortages of reagent.
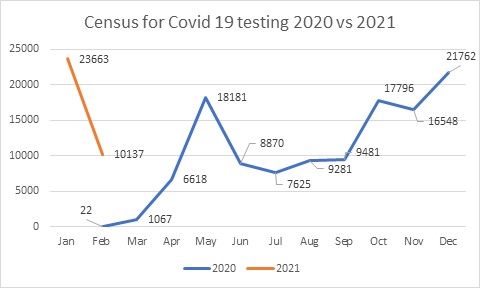
As of March 2021, our positivity rate is 0.72%. It has ranged from 0.03% to 3.7%, peaking in the months of May 2020, December 2020 and January 2021. It is going down at the moment.
We have considered three main factors managing supplies:
- Sufficiency of stocks
- Competitive pricing
- Quality of swabs and VTMs
We did try different brands of swab due to the issues on the break point and the quality of the swabs itself. As for the VTM we use a few brands in order to have sufficient stock level as well converted to VTM that required inactivation process as part of the testing protocol to inactivated VTM supplies.
A few unexpected things have happened thus far,
- Genomic contamination
- False negative/positive
- Internal control reliability – external vs human housekeeping genes
One of our continuing concerns is patients who obtain different test results when repeat swabs are done at different hospitals or Laboratories
- Based on our experience we noted, when the patient has very low levels of the virus or viral fragments, one day you might be positive. The next day, you might be negative. There's really nothing wrong with the test. It's just the function of the test.
- The lower the viral load, the less accurate the test would be.
When the viral titer is low, the process is subjected to sub-sampling error. The probability to pick up the same amount of virus is not guarantee and it could be not picking any at all or too little was picked up (out of assay LOD). Thus causing the discrepancy result. When low viral titer, sub-sampling per processed will be different. The repeat test on the single gene detected specimens with high CT value, most of the time it was not detected on the second run that cause the discrepancy result among the two tests. The reason why this scenario happened could be due to low titer of virus concentration or when the viral target is near to assay limit of detection (LOD) which in this case for our assay will be 100 copies RNA/ rxns. This means that to be call as detected we need to have minimum of 100 copies of SAR-CoV-2 RNA in the specimen.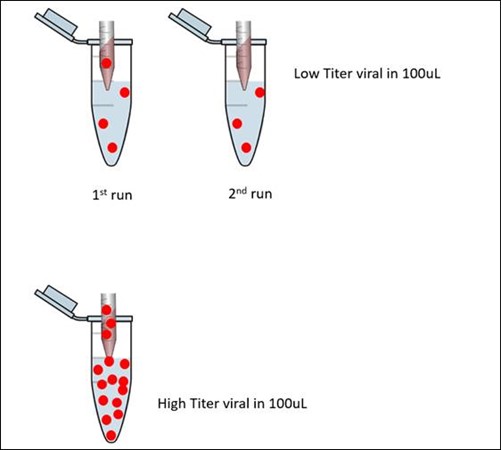
- The are many other variables to be considered for the RT PCR testing: sample was not collected during the virus shedding period, integrity of sample during transportation, inhibitors presence in the sample etc.
- Any results interpretation require clinical correlation with clinical history of the patient, signs and symptoms as well as the epidemiology link. Along the testing journey we learnt to understand the Kinetics of SARS-CoV -2.
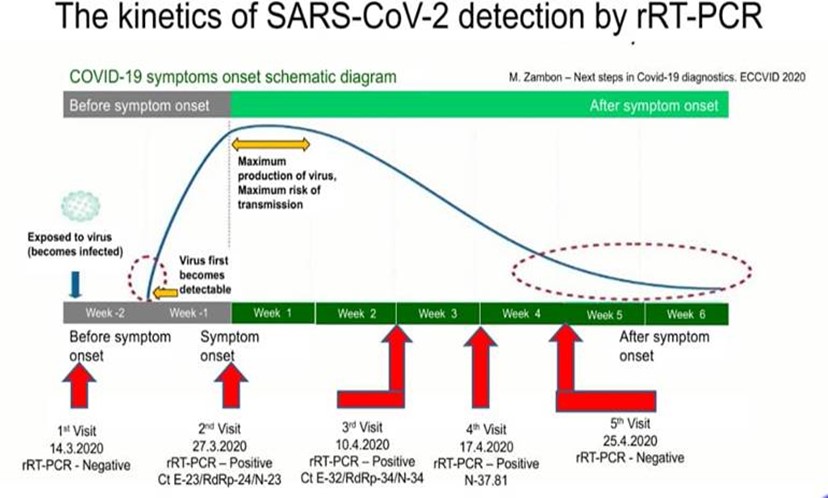
- An observation from one of the SunMed Consultant Microbiologists: If a patient has a Ct value of more than 33, if repeat swabbing are done, possibility is there to have negative results. Furthermore, if patients repeated swab test are done on different days, patient clinical condition may have differed as well.
One last comment on things where laboratories need more support
Generally, molecular testing process or workflow is quite manual indeed in terms of loading of orders in the extraction system as well as entering of results in Laboratory information system. We had to automate some of the result entry processes by scanning barcode ID with patient information and accession number, to reduce manual entry of the patient demographics in the analyzers. During the spike of the Covid 19 cases, a more streamlined processes with minimal intervention on results entry and verification would have been a great support.

We thank Sunway Medical Centre for sharing their experiences.