Sigma Verification of Performance Program
Innovative Diagnostics Sigma Verification of Performance
Innovative Diagnostics has become the first (and fastest) private laboratory in Singapore to achieve the Verification of Performance in May 2016. Further, it verified an additional branch (Camden) of the laboratory in September 2016. A total of more than 80 analytes, including chemistry, immunoassay, hormones and tumor markers on multiple instruments.
Innovative Diagnostics - Sigma Verification of Performance
Verified since 2016.
Re-Verified since August 1, 2019 through August 31st, 2020
Camden Office Duration of Verification: September 2nd, 2016 through October 1st, 2017
In May 2016, Westgard QC was pleased to verify the performance of more than 40 assays at Innovative Diagnostics Laboratory in Singapore.
In September 2016, Westgard was even more pleased to verify the performance of an additional branch of the laboratory. Then in early July 2017, Innovative re-verified his performance.

Innovative Diagnostics Pte Ltd is one of Singapore’s leading private pathology providers. Established in January 1996 as a subsidiary of SingHealth, Innovative officially changed hands to a private consortium led by doctors and a group of industry veterans in June 2011. Innovative Diagnostics has achieved the international gold standard for laboratory excellence by successfully obtaining CAP accreditation in 2013 and reaccreditation again in 2015. Currently, Innovative Diagnostics service over 1000 clients across Singapore comprising of general practitioners (GP), medical specialists, medical clinics, private hospitals, corporate clients, insurance companies, third party administrators and not for profit groups. For more information, please visit www.innovativelab.com.sg.
List of analytes verified
|
Assay |
Verified on ci162000 A 512726 |
Verified on ci162000 B 515206 |
|
Alkaline Phosphatase |
Six Sigma |
Six Sigma |
|
AST |
Six Sigma |
Six Sigma |
|
C-Reactive Protein |
Six Sigma |
Six Sigma |
|
Calcium |
Six Sigma |
Six Sigma |
|
Cholesterol, HDL |
Six Sigma |
Six Sigma |
|
Cholesterol, Total |
Six Sigma |
Six Sigma |
|
Creatine Kinase |
Six Sigma |
Six Sigma |
|
Creatinine |
Six Sigma |
Six Sigma |
|
Glucose |
Six Sigma |
|
|
GGT |
Six Sigma |
Six Sigma |
|
Iron |
Six Sigma |
|
|
Phosphorous |
Five Sigma |
|
|
Potassium |
Six Sigma |
Six Sigma |
|
Total Protein |
Six Sigma |
Six Sigma |
|
Sodium |
Four Sigma |
|
|
Triglycerides |
Six Sigma |
Six Sigma |
|
Uric Acid |
Six Sigma |
Six Sigma |
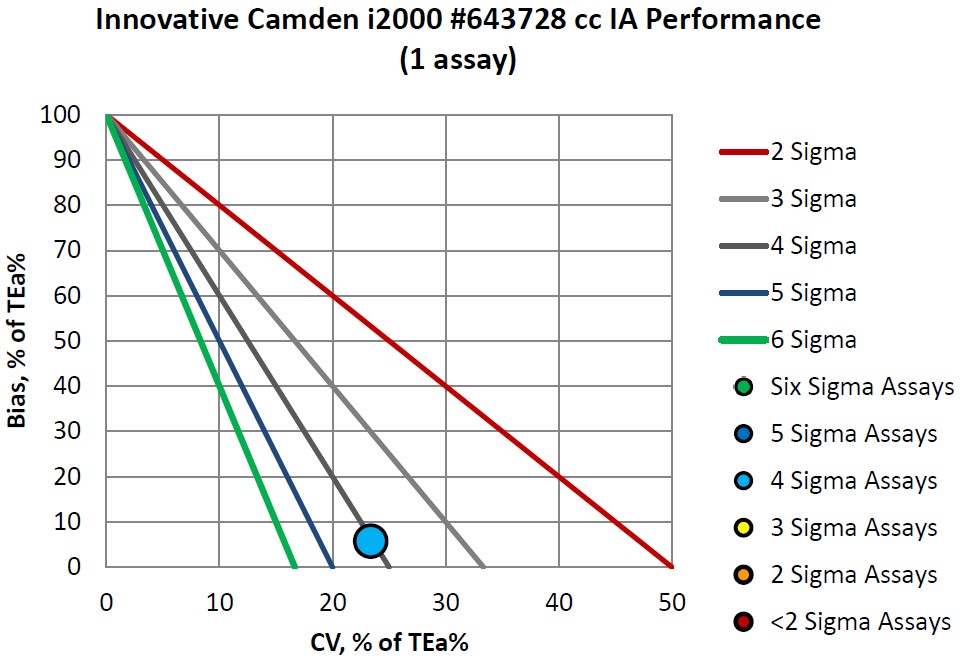
Here is the list of immunoassays:
|
Assay |
Verified on ci162000 A 512726 |
Verified on ci162000 B 515206 |
Verified on i4000 515218 |
|
CEA |
Four Sigma |
||
|
CA 125 |
Six Sigma |
Six Sigma |
|
|
LH |
Five Sigma |
||
|
PSA |
Five Sigma |
||
|
Vitamin B12 |
Four Sigma |
List of Expanded Camden Branch Analytes Verified:
Assay
| Assay |
Verified on ci8000 A |
Verified on ci8000 B |
|
Alkaline Phosphatase |
Six Sigma |
Six Sigma |
| Amylase | Five Sigma | |
|
AST |
Six Sigma |
Six Sigma |
|
Bilirubin, Direct |
Six Sigma |
Six Sigma |
|
Bilirubin, Total |
Six Sigma |
Six Sigma |
|
Calcium |
Six Sigma |
Four Sigma |
|
Chloride |
Six Sigma |
Six Sigma |
|
Cholesterol, HDL |
Six Sigma |
Six Sigma |
|
Cholesterol, Total |
Six Sigma |
Six Sigma |
|
Creatinine Kinase |
Six Sigma | Six Sigma |
|
Creatinine |
Six Sigma |
Six Sigma |
|
Glucose |
Six Sigma |
Five Sigma |
|
GGT |
Six Sigma |
Six Sigma |
| Iron | Six Sigma | |
|
Magnesium |
|
Six Sigma |
|
Phosphorous |
Five Sigma |
|
|
Potassium |
Six Sigma |
Six Sigma |
|
Total Protein |
Six Sigma |
Six Sigma |
|
Sodium |
Six Sigma |
|
|
Triglycerides |
Six Sigma |
Six Sigma |
|
Uric Acid |
Six Sigma |
Six Sigma |
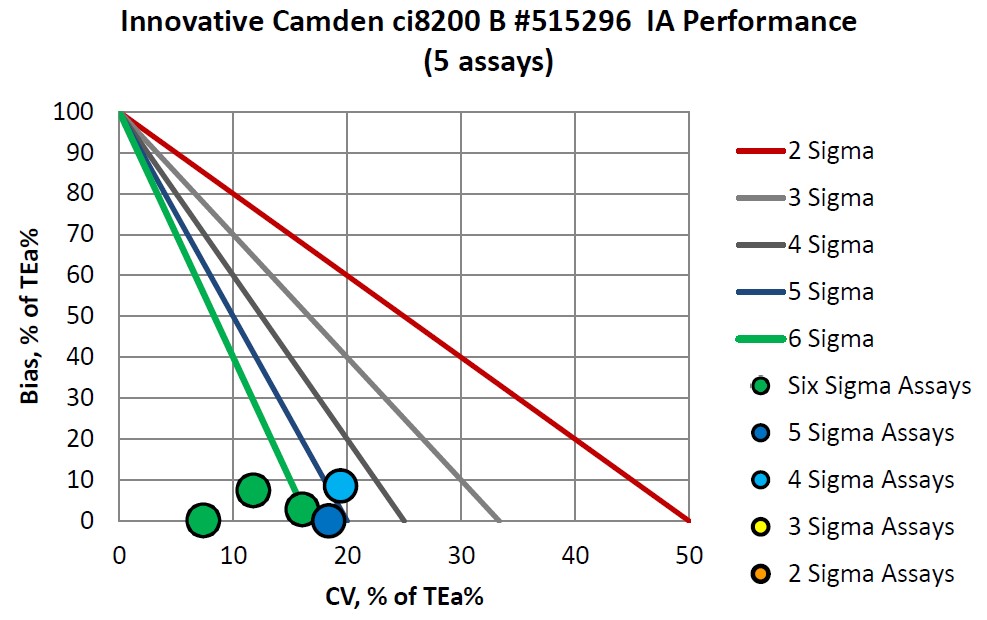
Immunoassays were also verified:
|
Assay |
Verified on ci8200 A |
Verified on ci8200 B |
Verified on i2000 C |
|
CA 125 |
Six Sigma |
Six Sigma |
|
|
CA 15-3 |
Four Sigma |
Four Sigma |
|
|
CEA |
Five Sigma |
Six Sigma |
|
|
PSA |
Five Sigma |
Five Sigma |
|
|
FSH |
Six Sigma |
||
|
LH |
|
Four Sigma |
|
|
Vitamin B12 |
|
|
Five Sigma |
Graphic Display of Performance of Core Lab Innovative Diagnostics
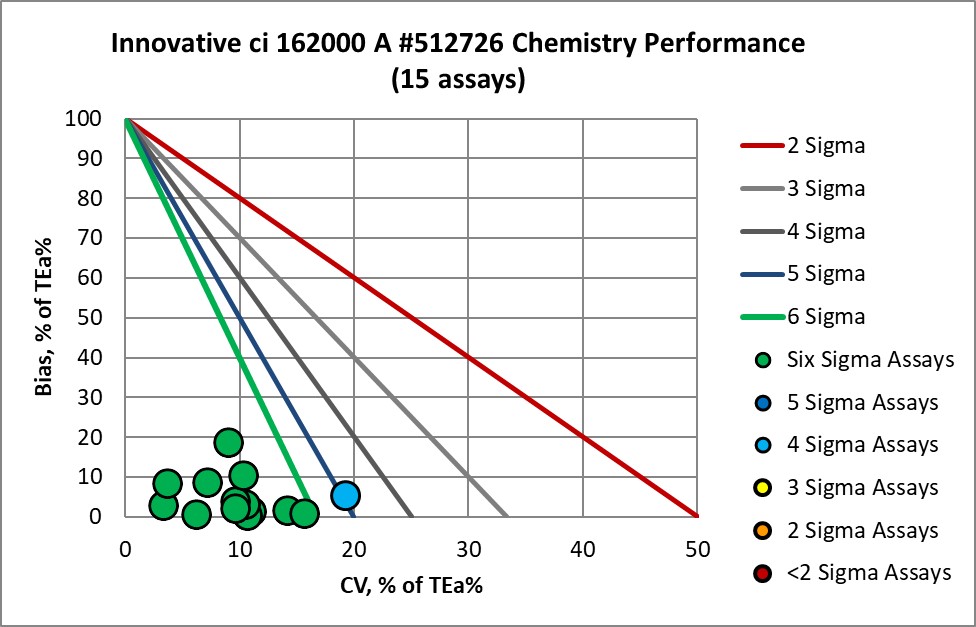
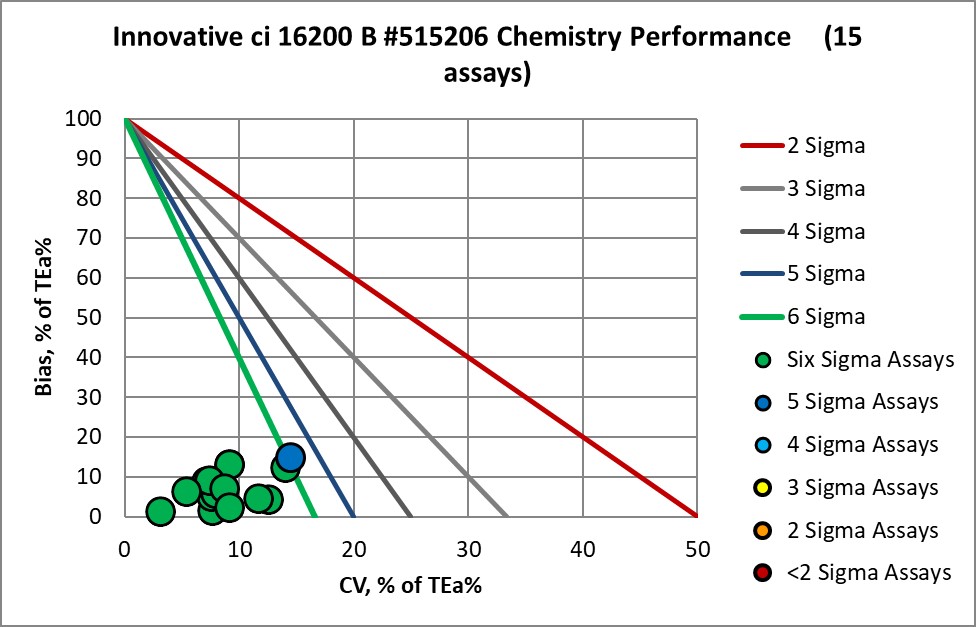
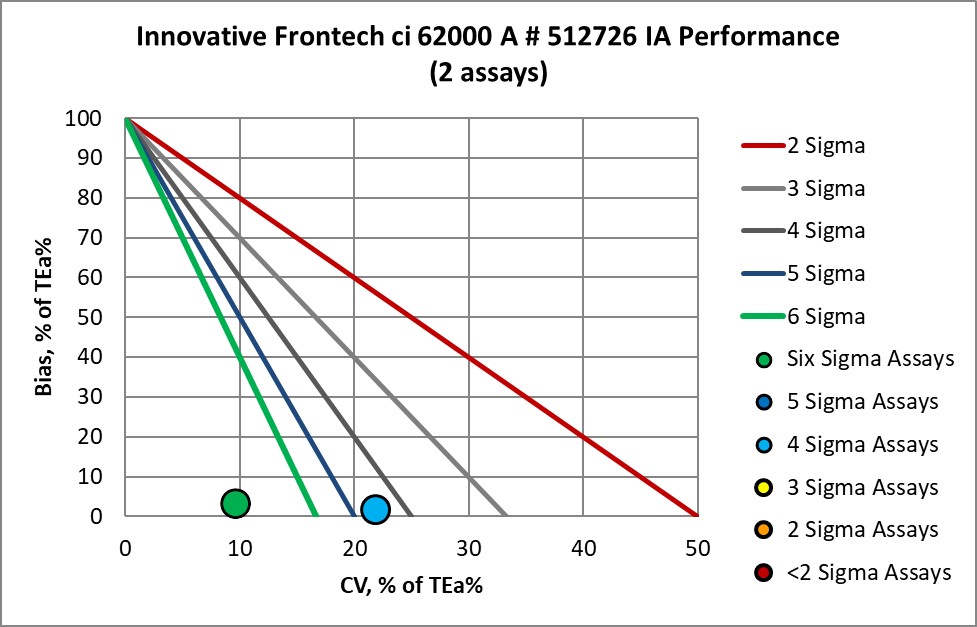
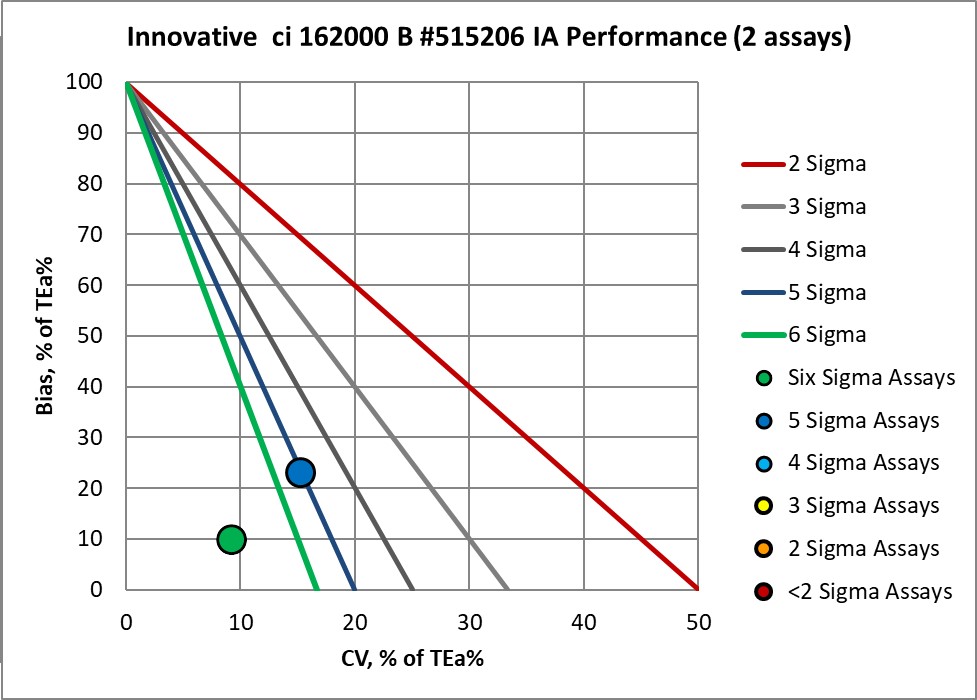
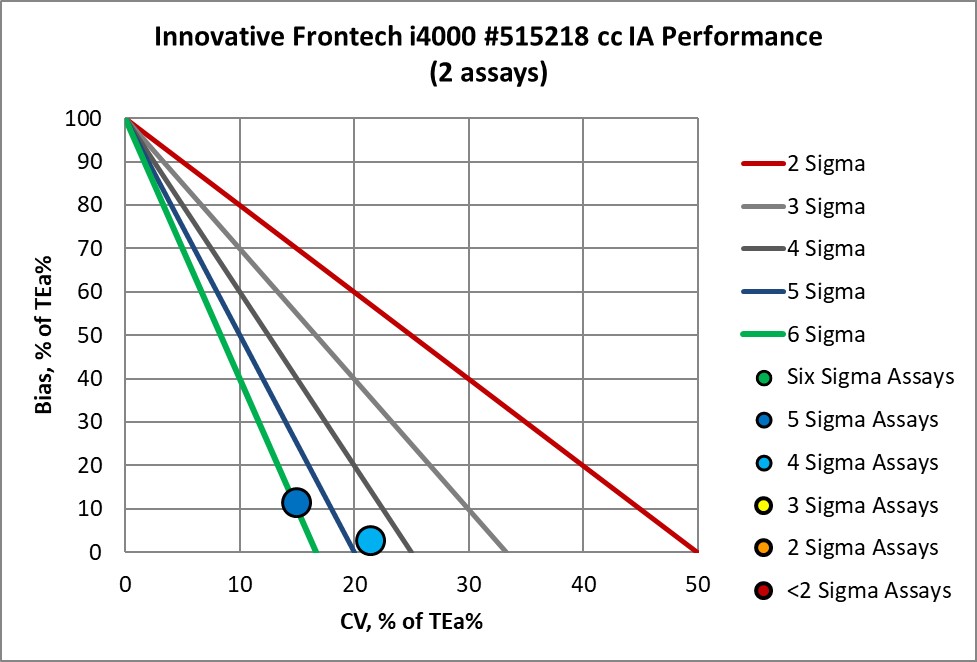
The Performance of the Expanded Branch of Innovative Diagnostics
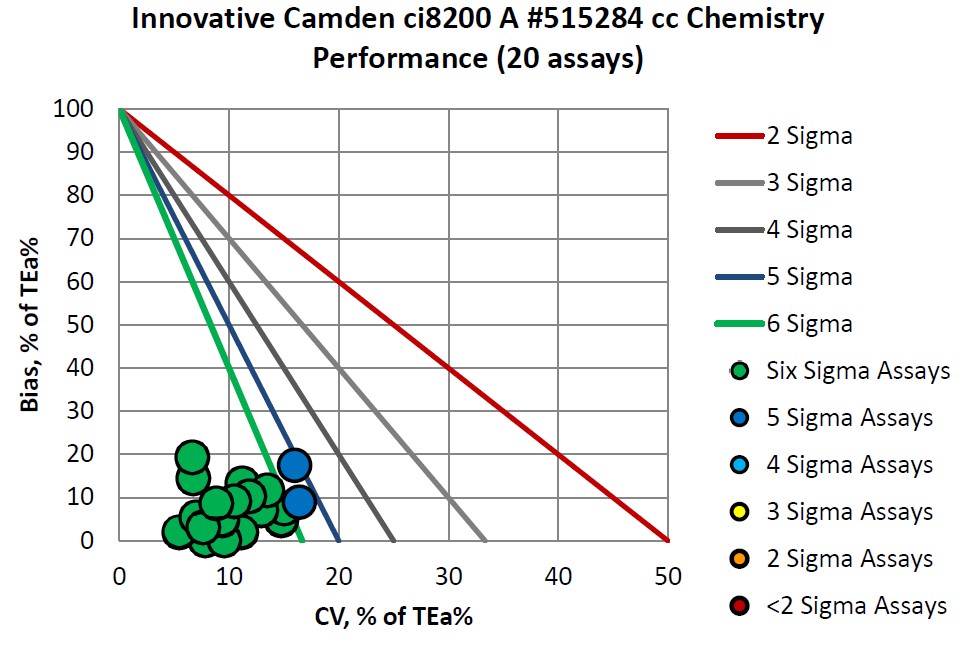
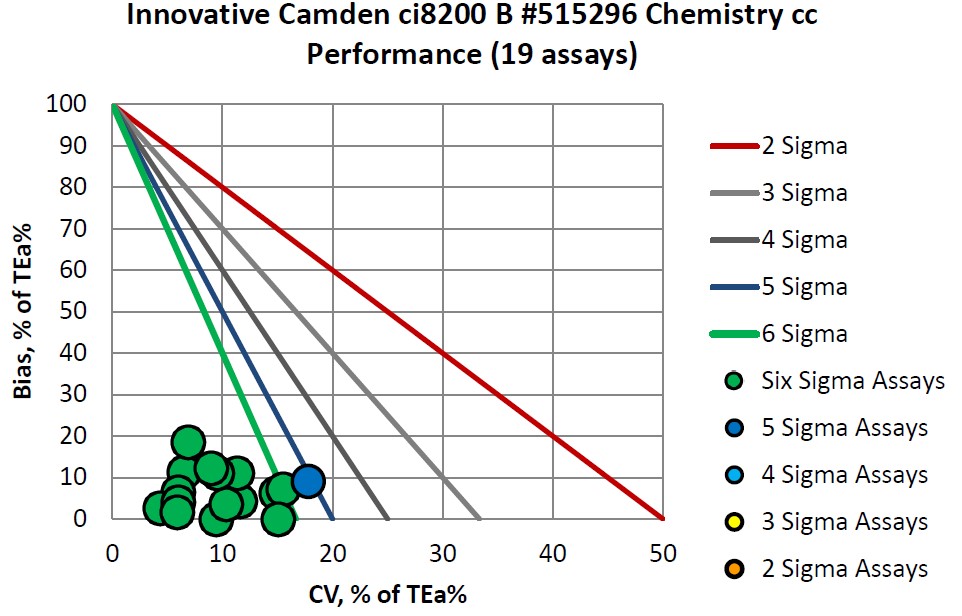
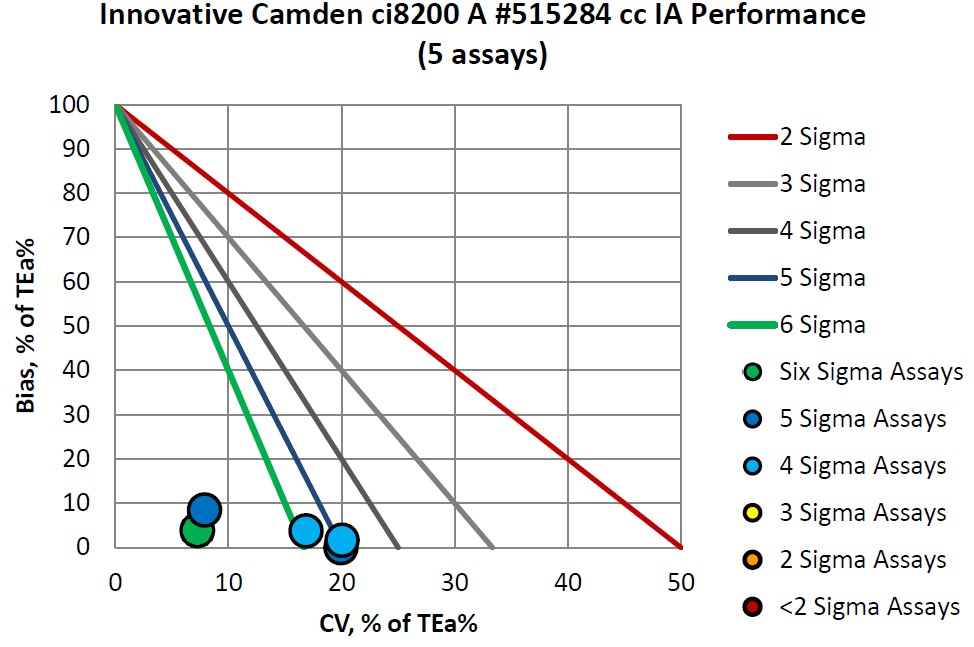
Congratulations to Innovative Diagnostics on their consistent accomplishments.



