QC Design
Mapping the Road to Analytical Quality
Quality is often described as a journey. Too often our efforts describe where we've been and how we arrived at the present, rather than advancing to where we need to be in the future. Laboratory efforts need to advance quality with well-defined destinations, maps to guide us to those destinations, and careful planning to provide a smooth journey. The way is revealed.
- Quality Planning
- Quality Requirements
- Quality Planning Models
- OPSpecs Charts
- QC Validator Program
- References
Quality is often described as a journey. Organizational change involved in most quality management programs may give the illusion of a journey, but it often represents only a temporary movement without any well-defined destination. Sometimes the effort mainly describes where we've been and how we arrived at the present, rather than advancing to where we need to be in the future. Laboratory efforts to advance quality need well-defined destinations, maps to guide us to those destinations, and careful planning to provide a smooth journey.
Quality Planning
The activities of defining the destination for the journey and mapping the path to that destination are part of quality-planning. The choice of destination often depends on the particular interests of the travelers involved, thus it is somewhat subjective and varies with the traveler. The path to a chosen destination should be objective, even though different travelers want to go to different destinations.
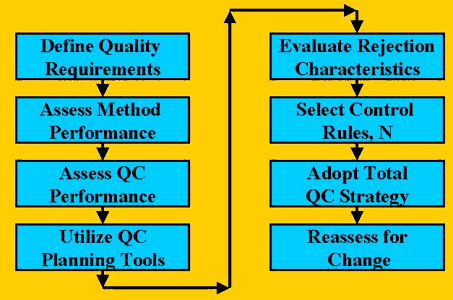 For quality planning in a laboratory, think of the destination as the quality desired or required for a test. Recognize that this quality requirement varies from laboratory to laboratory depending on the clinical services provided by the healthcare organization, the professional practices established, and the regulations enforced. This quality requirement must be defined to begin an objective quality planning process [1], as shown in the accompanying figure. The next steps include assessing the performance characteristics of the method, obtaining the rejection characteristics of the candidate QC procedures, selecting appropriate control rules and numbers of control measurements, adopting an overall or total QC strategy, and finally reassessing for changes if and when necessary.
For quality planning in a laboratory, think of the destination as the quality desired or required for a test. Recognize that this quality requirement varies from laboratory to laboratory depending on the clinical services provided by the healthcare organization, the professional practices established, and the regulations enforced. This quality requirement must be defined to begin an objective quality planning process [1], as shown in the accompanying figure. The next steps include assessing the performance characteristics of the method, obtaining the rejection characteristics of the candidate QC procedures, selecting appropriate control rules and numbers of control measurements, adopting an overall or total QC strategy, and finally reassessing for changes if and when necessary.
The planning activity itself may be supported by different tools and technology. For example, one well-known travel planning organization in the US (AAA, American Automobile Association) offers guide books, regional and state maps, and "travel-ticks" (very detailed step by step maps). New technology is also emerging in the form of personal computer programs for mapping, on-line and interactive maps on the World Wide Web, and on-board guidance systems. All these planning tools and technology still require knowledge of the destination (quality requirement), as well as the starting point (method performance characteristics, QC rejection characteristics). However, they may use this information in different ways to develop a plan for the journey.
The practical details of how quality planning can be performed in a laboratory depend on the particular QC planning tools and technology that are utilized. These may include basic tools such as power function and critical-error graphs [2], more advanced tools such as quality-planning models [3,4] and OPSpecs charts [5,6], and even computer technology, such as the QC Validator 2.0 program that automates the selection of QC procedures [7,8]. On- board guidance systems are not yet available, but that will be the technology of the future for managing the analytical quality of automated systems.
Quality requirements
Destinations are provided in the form of goals, objectives, and requirements that give direction to our planning, our policies, our processes, and our procedures, as well as to our people who are involved in carrying out the mission of the laboratory. For analytical quality, destinations are the quality requirements that define acceptable laboratory performance. To arrive at these destinations requires directions for the precision, accuracy, and QC needed for testing processes to operate satisfactorily at the bench level.
Quality requirements in the form of analytical total errors or clinical decision intervals are advantageous because they can be translated into specifications for the precision and accuracy that are allowable for the method and the QC that is necessary to detect changes in method performance. Other kinds of quality requirements are, in a sense, incomplete because they lack information about the QC necessary to assure satisfactory performance in routine operation. However, they can sometimes be reformulated into an allowable total error requirement to make it possible to include quality control [10].
Quality-planning models
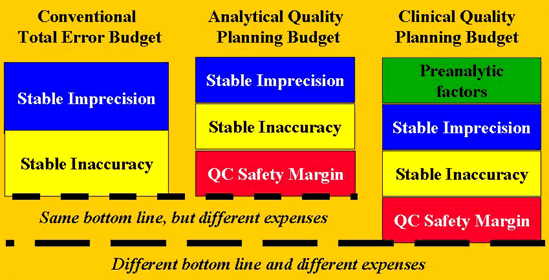
Quality-planning models are used to define the relationship between an allowable total error or a medically significant change and the analytical and preanalytical factors that contribute to the variability of a test result.
These models are "error budgets" that describe the factors being considered in controlling or managing the variability of a test result. Most of our current thinking about analytical quality management is based on the conventional total error budget that considers only the effects of stable imprecision and stable inaccuracy. The analytical quality planning model brings in the additional consideration of the QC sensitivity needed to detect changes in stable method performance. As can be seen in the accompanying figure, the bottom line is the same as for the conventional total error budget, however, better method performance is needed to guarantee that the desired quality is achieved in everyday operation. The clinical quality planning model brings in the additional effects of preanalytical factors, such as within-subject biological variation, which means there is a different bottom line as well as additional expenses to be considered.
OPSpecs charts
Practical maps for guiding laboratories to defined quality destinations can be provided in the form of charts of operating specifications (OPSpecs charts), which can be prepared for quality requirements in the form of analytical total errors or clinical decision intervals. The quality-planning models are displayed by showing the allowable inaccuracy on the y-axis and the allowable imprecision on the x-axis for different QC procedures (control rules, numbers of control observations) that provide a defined level of error detection (the quality requirement, or analytical quality assurance).
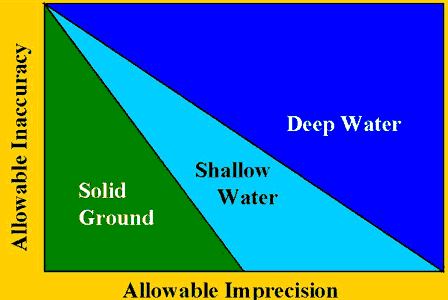
The analogy of the OPSpecs chart to a map helps describe its use. As shown in the accompanying figure, the map outlines the boundaries between deep water, shallow water, and land.
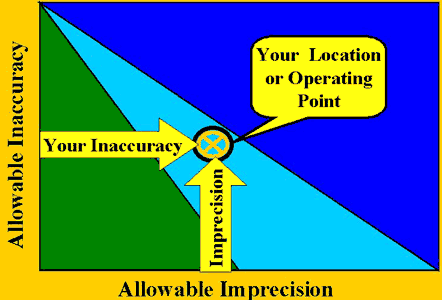
The location of a given method is determined by plotting its inaccuracy on the y-axis and its imprecision on the x-axis to define the operating point. The objective is to be on solid ground, i.e., to be located within the allowable imprecision and allowable inaccuracy of a candidate QC procedure, which vary with control rules and number of control measurements. Furthermore, if you're not on solid ground, you can easily see what changes in method performance are desirable to move out of the water and onto dry land.
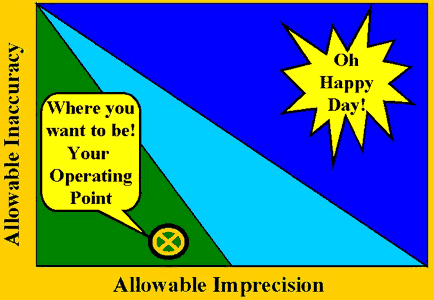
Three situations will be encountered when using OPSpecs charts. In the best case, the method has low enough imprecision and inaccuracy to be easily controllable, as shown in the accompanying figure. In this case, the main effort should be to minimize the cost of quality control by reducing the number of control measurements to the minimum required, widening the control limits to minimize false rejections, and simplifying the control rules to make the QC procedure as easy as possible to implement.
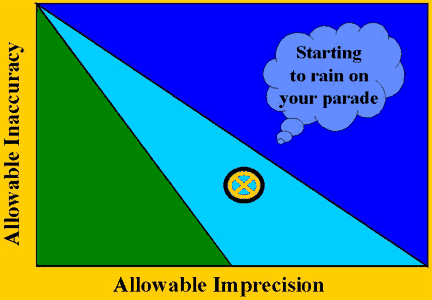
In the middle case, as shown by the next figure, the method's imprecision and inaccuracy will provide acceptable performance during stable operation, but the method will not be controllable if problems occur and operation becomes unstable. It may be possible to improve the QC procedure by increasing the number of control measurements and using multi-rule rather than single-rule procedures. Most likely it will also be necessary to improve method performance by eliminating any bias and reducing the standard deviation.
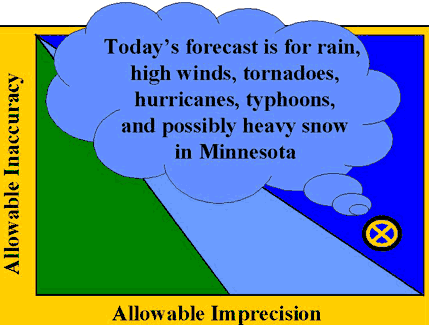
In the worst case, as shown by the last figure, the method doesn't even provide the necessary quality when performance is stable. How to do QC is not the right question. What method to use is the more relevant question. While it is possible that method performance can be improved, it may actually be necessary to replace this method with newer and better technology to achieve the necessary analytical performance.
These OPSpecs maps are available in the scientific literature [5], on the world wide web, and in printed form in an OPSpecs Manual [9]. You can think of the OPSpecs Manual as an atlas, with the quality requirement being a "state" or "county." Think of the operating point as the coordinates within that state. You define the state of interest, lookup the map for that state, then find your location on that map. The OPSpecs Manual contains maps for a wide range of states, from 0.5% to 50%, which include all those necessary for the full range of requirements specified by the US CLIA proficiency testing criteria for acceptable performance.
QC Validator Program
OPSpecs charts can be prepared with a specialized PC computer program, QC Validator (now EZ Rules 3), which runs under the Windows operating system. The program can prepare OPSpecs charts for both analytical total error quality requirements and clinical decision interval quality requirements. Version 2.0 offers an automatic QC selection process that selects the control rules and numbers of control measurements appropriate for the input parameters (quality requirement, method imprecision, method inaccuracy) and certain logic and criteria (types of rules, total number of control measurements, desired probabilities for false rejection and error detection) that are editable by the user. This automatic selection process provides a key component of the technology needed to totally automate the quality control process.
Conclusion
If you are disappointed by the lack of technical details in this discussion, let me assure you that the details are already available in the literature. The references included here are only a part of what has been published on this approach. My experience in teaching QC planning is that too many details will initially overwhelm the ideas and concepts, thus it is important to first grasp the ideas and gain an understanding of the conceptual approach. Then the details will become relevant and you will be motivated to learn them. This presentation is a test of that hypothesis and you can assess its significance.
Analytical quality is, of course, only part of the needs and requirements for laboratory testing. However, Analytical quality is fundamental to the core laboratory process, which is providing reliable diagnostic information. If we are unable to do that, the rest doesn't really matter.
Many will argue will analytical quality is already good enough, and we need to focus on the "rest." There are two difficulties with this opinion. First, the question, "What quality is necessary?" must be answered, and the truth of the matter is that few laboratories actually define the quality they want to achieve -- how do we know the quality is good enough if we have no definition for what "good enough" actually means? Second, this conclusion is based on an oversimplified total error model that does not adequately consider the test result in everyday operation. My answer to both questions is to ask you to go through the QC planning process for all the methods in your laboratories and see if they really are good enough. The tools and technology are now available to make it practical to perform this performance assessment for all the tests in your laboratories.
Concerning the "bigger" issues of quality and the new approaches to quality management, the ideas and concepts discussed here still apply. Just describing what you're doing in your laboratory and writing it down does not constitute quality planning. That's just arbitrary quality! To manage or control quality, we need to define the destinations (or requirements) for each performance characteristic. If you don't know the destination, how can you manage to get there? Where are the maps to guide you and make the journey smooth? Managing quality depends on having specifications that tell us exactly what needs to be done at the bench level to guarantee our services are delivered with the necessary quality. Goals and requirements must be translated into operational specifications if we are to achieve quality in laboratory services.
References
- Westgard JO. Error budgets for quality management: Practical tools for planning and assuring the analytical quality of laboratory testing processes. Clin Lab Manag Review 1996;10:377-403.
- Westgard JO, Groth T. Power functions for statistical control rules. Clin Chem 1979;25:863-69.
- Westgard JO, Hytoft Petersen P, Wiebe DA. Laboratory process specifications for assuring quality in the U.S. National Cholesterol Education Program (NCEP). Clin Chem 1991:37:656-661.
- Westgard JO, Wiebe DA. Cholesterol operational process specifications for assuring the quality required by CLIA proficiency testing. Clin Chem 1991;37:1938-44.
- Westgard JO. Charts of operational process specifications ("OPSpecs charts") for assessing the precision, accuracy, and quality control needed to satisfy proficiency testing criteria. Clin Chem 1992;38:1226-33.
- Westgard JO. Analytical quality assurance through process planning and quality control. Arch Pathol Lab Med 1992;116:765-769.
- Westgard JO, Stein B, Westgard SA, Kennedy R. QC Validator 2.0: a computer program for automatic selection of statistical QC procedures in healthcare laboratories. Comput Method Program Biomed 1997;53:175-186.
- Westgard JO, Stein B. An automatic process for selecting statistical QC procedures to assure clinical or analytical quality requirements. Clin Chem 1997;43:400-403.
- Westgard JO. OPSpecs Manual - Expanded Edition. 1996;Westgard Quality Corporation, Ogunquit, ME.
- Hyltoft Petersen P, Ricós C, Stöckl D, Libeer J-C, Baadenhuijsen H, Fraser CG, Thienpont L. Proposed guidelines for the internal quality control of analytical results in the medical laboratory. Discussion paper from the members of the external quality assessment (EQA) working group A on the analytical quality goals in laboratory medicine. Eur J Clin Chem Clin Biochem 1996;34:983-99.
