Sigma Verification of Performance Program
Sigma Verification of Tumor Center Lab of Cho Ray Hospital
The laboratory of the Cho Ray hospital in Ho Chi Minh City, Vietnam has successfully met the standards of the Sigma VP program since 2017.
Sigma Verification of the Tumor Center Laboratory of the Cho Ray Hospital
Original Verification: December 11th, 2017
Duration of Verification: September 1, 2020 through October 31st, 2021
"Chợ Rẫy Hospital is the largest general hospital in Ho Chi Minh City, Vietnam, founded in 1900 during the French colonial rule as Hôpital Municipal de Cholon. Over the years, the hospital has also been known as Hôpital Indigène de Cochinchine (1919), Hôpital Lolung Bonnoires (1938), and Hôpital 415 (1945), until it was ultimately renamed Chợ Rẫy in 1957.The facility was reconstructed on the area of 53,000 m² and was re-equipped to become one of the largest hospitals in Southeast Asia in June 1974At present, the hospital has 35 clinical, 11 subclinical and 8 functional departments. It organizes practice and postgraduate training for more than 2,500 medical students and 600 doctors each year. Chợ Rẫy Hospital has 1200 beds, employs 2,270 health workers including 500 medical doctors and pharmacists, and provides treatment for about 457,000 outpatients and 67,000 inpatients per year."
The following assays and instruments which were verified
|
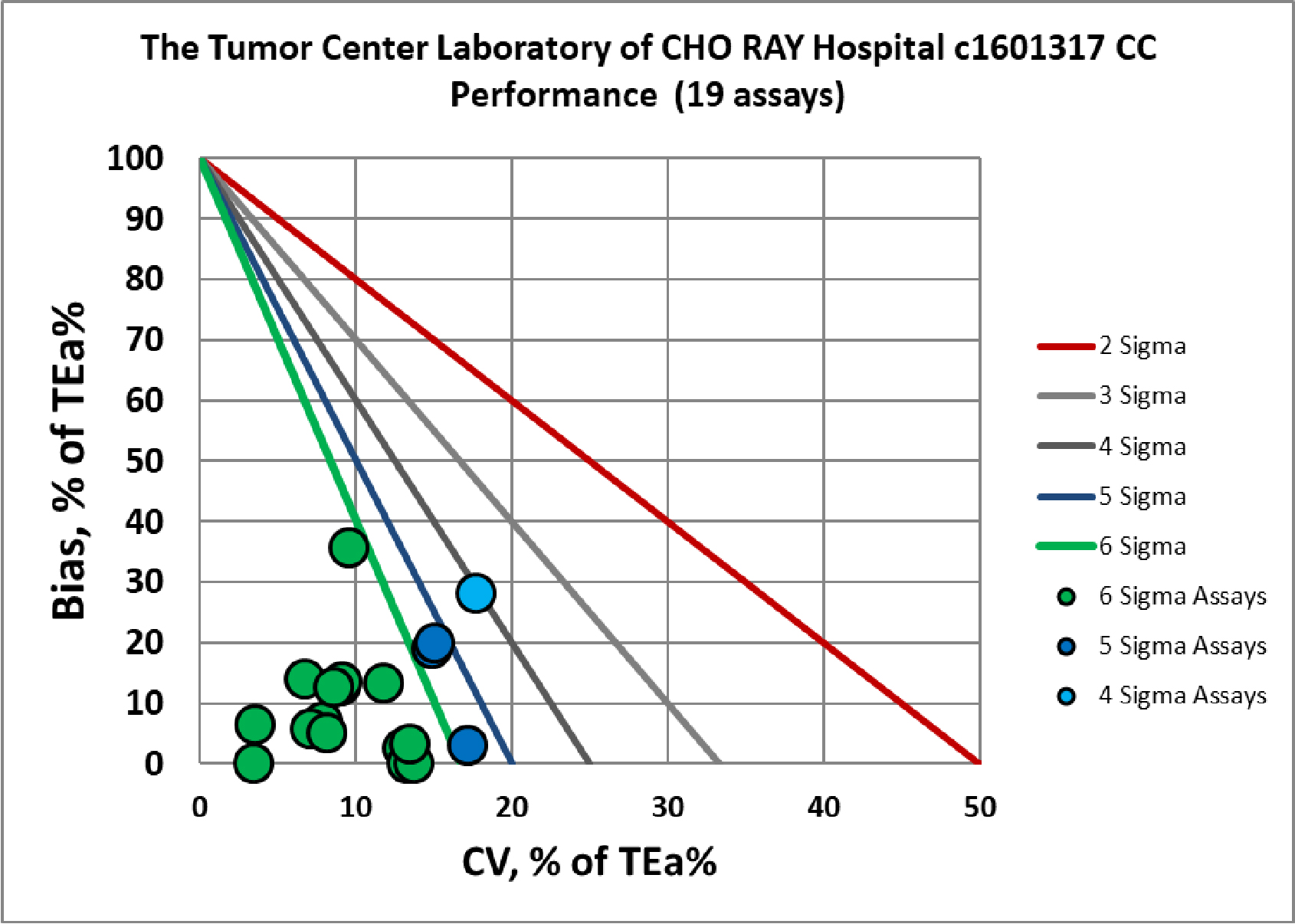
Chemistry Assay |
Verified c1601318 |
Verified c1601317 |
|
Albumin |
Six Sigma |
Six Sigma |
|
ALT |
Six Sigma |
Six Sigma |
|
AST |
Six Sigma |
Six Sigma |
|
Bilirubin, Direct |
Six Sigma |
Six Sigma |
|
Bilirubin, Total |
- |
Six Sigma |
|
C Reactive Protein |
- |
Six Sigma |
|
Calcium |
Six Sigma |
Six Sigma |
|
Chloride |
Four Sigma |
- |
|
Cholesterol |
Six Sigma |
Five Sigma |
|
Creatinine |
Six Sigma |
Six Sigma |
|
GGT |
Six Sigma |
Six Sigma |
|
Glucose |
Four Sigma |
Five Sigma |
|
Iron |
Four Sigma |
Five Sigma |
|
HDL |
Six Sigma |
Six Sigma |
|
LDL |
Six Sigma |
Six Sigma |
|
LDH |
- |
Four Sigma |
|
Magnesium |
Six Sigma |
Six Sigma |
|
Potassium |
Six Sigma |
Six Sigma |
|
Triglycerides |
Six Sigma |
Six Sigma |
|
Uric Acid |
Five Sigma |
Six Sigma |
in addition, immunoassays on multiple instruments we re-verified
|
Immunoassays |
Verified i2000 (multiple instruments) |
|
AFP |
Five Sigma |
|
CA 19-9 |
Six Sigma |
|
CA 125 |
Six Sigma |
|
PSA |
Six Sigma |
|
LH |
Four Sigma |
|
Hematology |
Verified on Alinity hq 270 |
|
HGB |
Six Sigma |
|
MCV |
Six Sigma |
|
MCH |
Five Sigma |
|
Platelets |
Six Sigma |
|
RBC |
Four Sigma |
|
WBC |
Six Sigma |
|
Lymphocytes |
Four Sigma |
|
Neutrophils |
Six Sigma |
Six Sigma: World Class Quality. Possible to use 3s to 3.5s limits and a minimum of controls
Five Sigma: Excellent Quality. Possible to use 3s limits or a 1:3s/2:2s/R:4s Westgard Rule
Four Sigma: Good Quality. Possible to use 1:3s/2:2s/R:4s/4:1s Westgard Rule
Visual Summary of Performance of Cho Ray Tumor Center's Laboratory
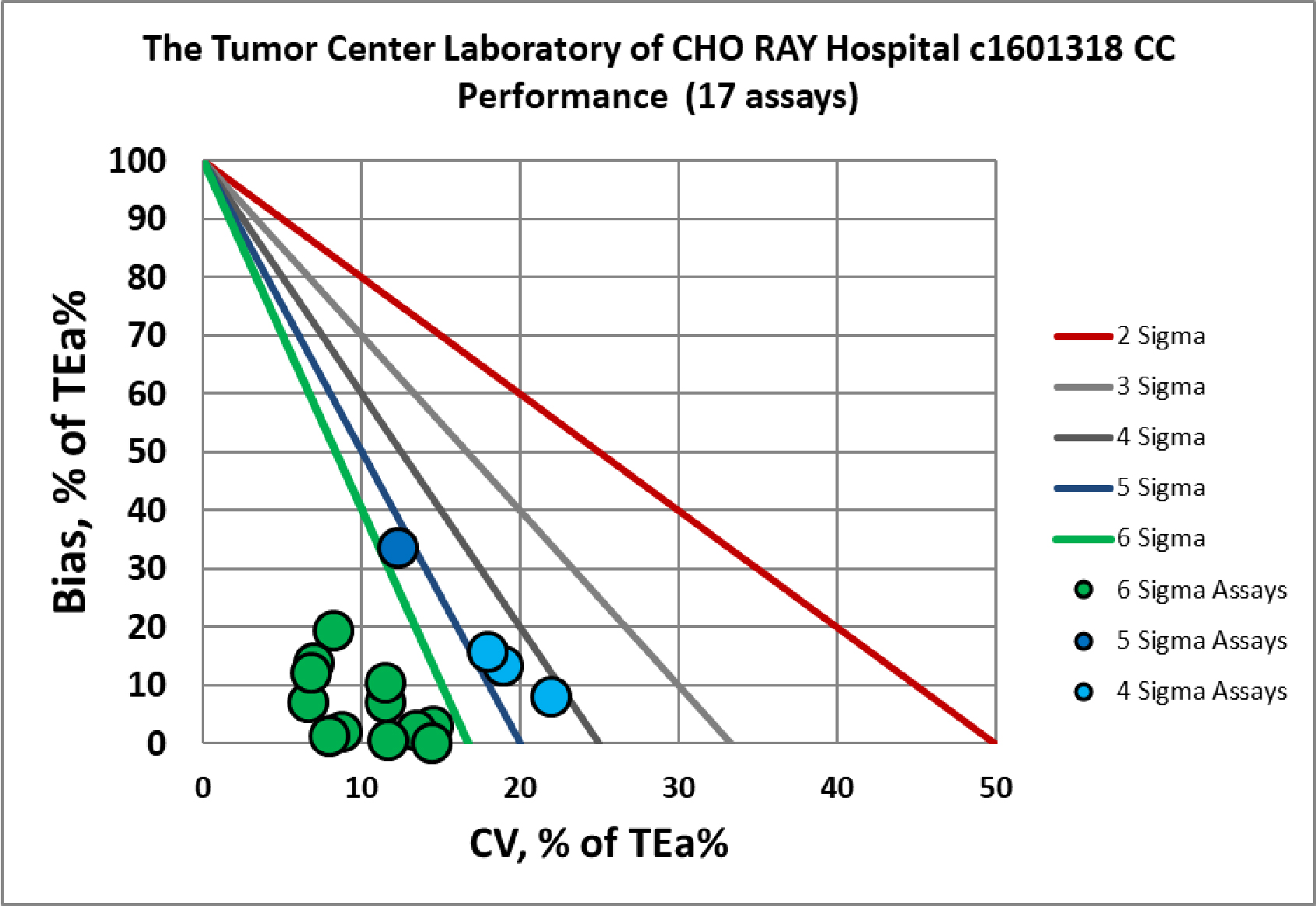
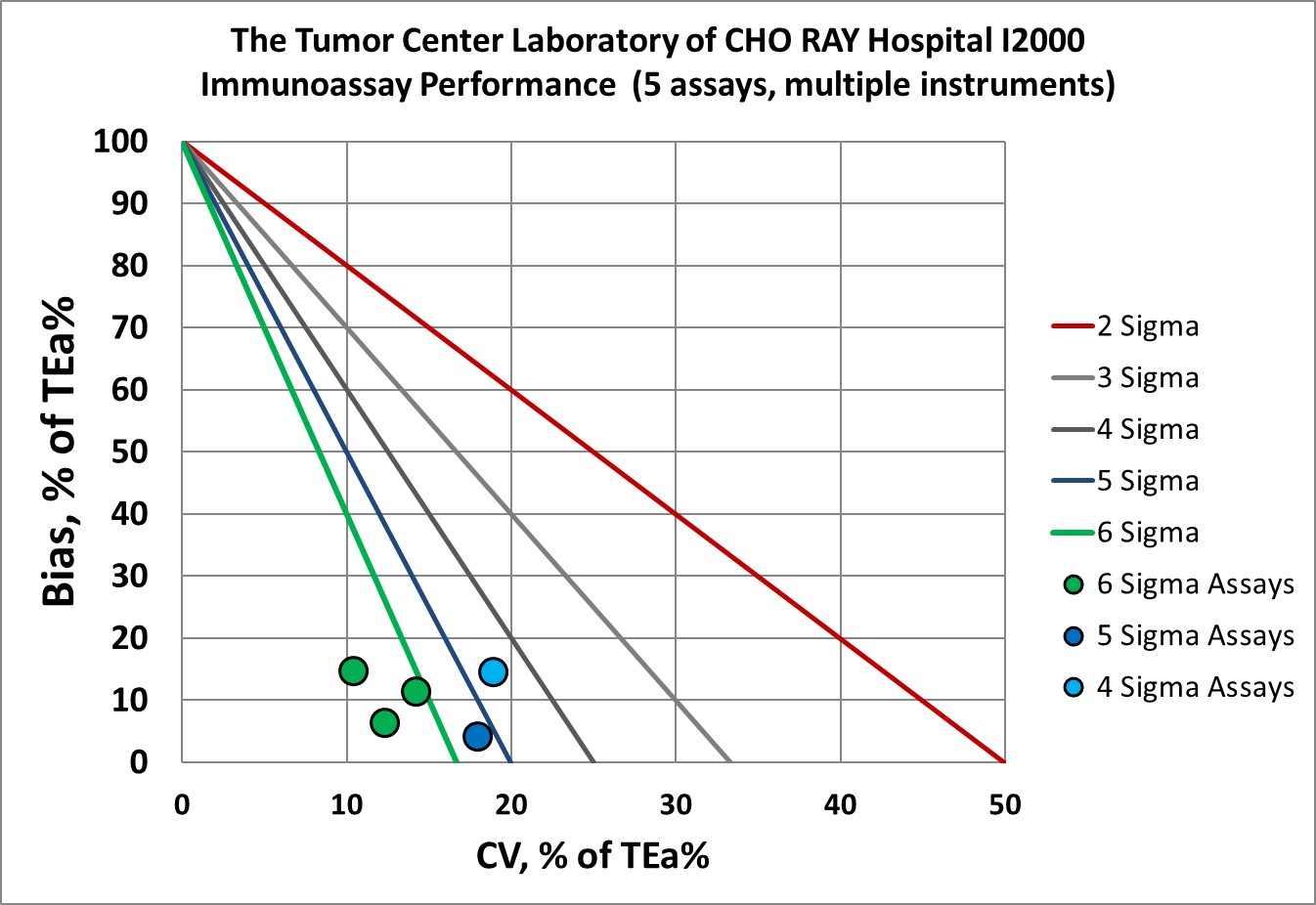
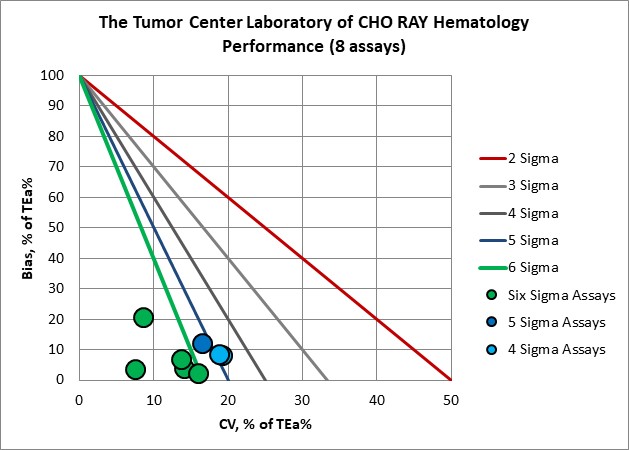
Congratulations to the entire Tumor Center Laboratory staff of the Cho Ray hospital.