Sigma Metric Analysis
Alinity HbA1c method in US, multimode analysis
Our first analysis of 2024 tackles a cobas 6000 in Turkey. Can the instrument meet CLIA 2024 standards, EFLM minimum, or EFLM desirable performance specifications?
Alinity HbA1c method in the US, multimode analysis
March 2024
Sten Westgard, MS
See the other analyses in this series:
- Beckman Coulter DxC 700
- Abbott Alinity
- Siemens Atellica
- Siemens Atellica in Romania
- Siemens Atellica in Spain
- Siemens ADVIA 2120i
- Roche c501 in Turkey
- Roche c501 in Saudi Arabia
- MicroLab RX-50 in India
- Roche cobas 6000 immunoassays in Turkey
- Sysmex XN 350 in India
- Dymind DH 76 in Bulgaria
- Mindray 7500 in China
- Mindray BS 2000M in China
Sigma Metric Score: 3.5 out of 6. Moderate quality. [Biological variation goals utilized. Biological goals used for assessment. Bias was assessed comparing against a reference (HPLC) method, using patient samples.]
This Alinity HbA1c method comes from the University of Kansas Medical Center:
Technical Note: Comparison of Alinity c Hemoglobin A1c Immunoassay with Premier Hb9210 Automated HPLC Assay: A preliminary report. Walton A, Meador J, Woodard K, Tucker S, McIntyre S, Dasgupta A. Annals of Clinical & Laboratory Science, vol 54, no. 1, 2024.
The total imprecision measured for the Alinity HbA1c method was assessed following the CLSI EP 05-A2 guideline, with 2% CV at an HbA1c level of 6.5%, and 3.5% CV at the level of 7.0%. The level of 6.5% is particularly critical, as this is where diabetes is diagnosed.
The bias was determined by comparison of methods with 77 whole blood speciments to the Premier Hb9210, an HPLC reference method. The regression equation was determined to be y = 0.9483x + 0.1548.
There exist a variety of performance specifications for HbA1c. The EFLM biological variation database sets the desirable total error goal at 2.4% and the minimum at 3.6%. The NGSP goal is set at 6.0%. Then the new CLIA 2024 goal, not without controversy, is set at 8.0%
With these pieces of data, Sigma metrics can be calculated and the results plotted on a normalized method decision chart (NMEDx).
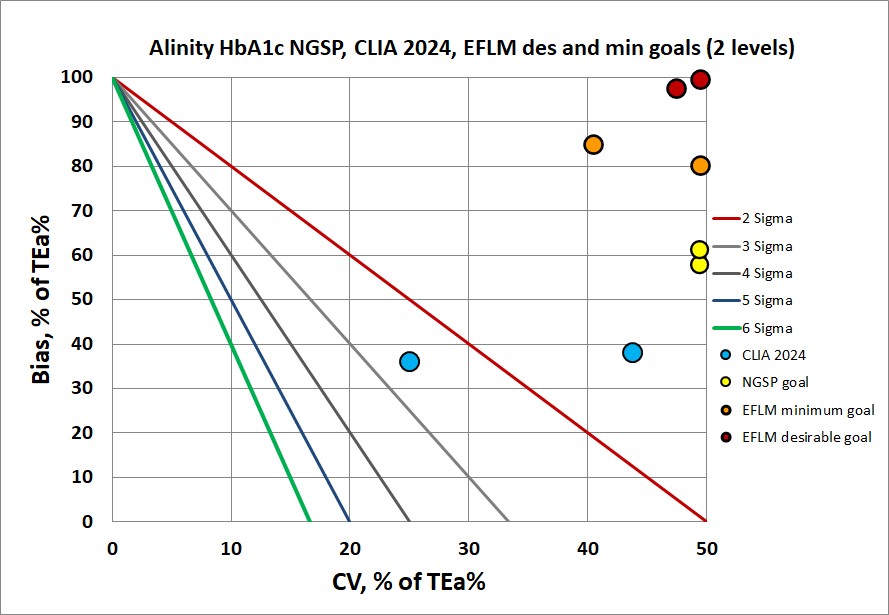
This is a disheartening picture. Even with the most forgiving goal (CLIA 2024), the method cannot achive even 3 Sigma. While bias appears to be the biggest problem, imprecision is also high.
Conclusion
The authors provide a compelling judgment: "The regression equation indicates negative bias in the HbA1c assay using Alinity c analyzer compared to the HPLC method. This may be problematic at decision point of HbA1c above 6.4% as well as 7%....[A]t the >6.4% decision point HbA1c values obtained by the Alinity c analyzer was on average 2.8% lower compared to the corresponding values obtained by the HPLC method....Therefore, values obtained at the decision points must be interpreted with caution and if clinically indicated should be reassessed using a HPLC method."
