ISO
ISO 15189:2012 Medical laboratories - Requirements for quality and competence
Contributing editor Dr. Pereira continues part 2 of a series on the ISO standards applicable to medical laboratories. The ISO 15189 is widely popular for laboratories, but many aspects are confusing, vague, and misunderstood. Dr. Pereira shows how to interpret the standard's requirements.
ISO series update
Part 2 - ISO 15189:2012 “Medical laboratories - Requirements for quality and competence”
Paulo Pereira, PhD
February 2017 Updated January 2020
Recap
ISO standards are intended to standardize practices globally. Unfortunately ISO implementation is frequently accompanied by misunderstandings. This series discusses the pros and cons, and some myths regarding the ISO standards’ implementation in medical laboratories. It will be divided into five parts:
- Part 1 - ISO 9001:2015 “Quality management systems - Requirements”
- Part 2 - ISO 15189: 2012 “Medical laboratories - Requirements for quality and competence”
- Part 3 - ISO 10012:2003 “Measurement management systems - Requirements for measurement processes and measuring equipment”
- Part 4 - ISO 19011 “Guidelines for auditing management systems”
- Part 5 - ISO 15190: 2003 “Medical laboratories - Requirements for safety”
Occasionally, the reader of an ISO standard may be challenged to identify what is mandatory and what is not required. ISO 9001 makes the reader’s life easier by noting in the introduction that “shall” specifies a requirement, “should” specifies a recommendation, “may” specifies a permission, and “can” specifies a possibility or a capability. Laboratories must to all “Shalls” but all the others are not mandatory.
Purpose
This text is probably the most challenging on the “ISO series update” since many reviews have been published related to ISO 15189 3rd edition [1]. Therefore, this essay is intended to discuss some specifications briefly and to debate what is happening with ISO 15189 implementation in the world. The standard document is focused on the medical laboratory, and its goals can be interpreted as the satisfaction of interested parties (4 of [1]). Stakeholders cannot be understood as customers only, such as patients, but inclusive of any internal or external involvement with the medical laboratory, including, but not only, professionals, suppliers and accreditation agencies. “The customers’ satisfaction” can be understood as the contribution of the reported results to an accurate clinical decision. For this purpose, this international standard is based not only on a management system but also on a set of medical laboratory technical specifications. Despite the lab sustainability should be controlled, it is not mandatory. Such as common in others ISO standards, “shall” stipulates a requirement, “should” specify a recommendation, “may” instructs permission, and “can” suggests a possibility or a capability. Only the “shall” signifies that a particular specification is compulsory.
The approach
ISO Technical Committee (TC) 212 has formed the ISO 15189 (Medical laboratory testing and in vitro diagnostic test systems) Working Group (WG) 1 (Quality and competence in the medical laboratory). The ISO 15189 debut edition [2] was published after a three-year hiatus from the final draft and four years after the publication of ISO/IEC 17025 first edition [3]. It quickly became a widely-accepted standard to be used for accreditation of medical laboratory competence. The 2nd edition [4] was published in 2007 to provide the same structure as ISO/IEC 17025, intended for testing and calibration in general laboratories. ISO 15189 can be viewed as the “ISO/IEC 17025” for medical laboratories. When a medical laboratory chooses an accreditation plan, it should select an accrediting body that operates according to appropriate international standards and which takes into account the particular requirements of this field. Before 2003 medical laboratories could be accredited according to the ISO/IEC 17025 approach, and they were able to change to ISO 15189 at their option. Depending on the local accrediting bodies it may be possible for a medical laboratory to choose between ISO/IEC 17025 and ISO 15189 or even to have both accreditations. For instance, when a medical laboratory has ISO 15189-accredited tests and also has a calibration method intended to calibrate not only internal devices (which does not require an ISO/IEC 17025 accreditation) but also equipment for external customers.
The difference between ISO 9001 and ISO 15189 approach that is immediately recognized is the presence of medical-technical laboratory requirements in ISO 15189. The framework provides a quality management system close to the ISO 9001:2008 management requirements added by specifications for technical competence that are particular to medical laboratories.
The standard quality management model is based on the Deming TQM approach [5] [6] [7]. Figure 1 displays a quality cycle applicable to a medical laboratory under ISO 15189 accreditation. The leadership is critical to the success of all the cycle phases. Probably, on the next guideline revision, “customers” will be replaced by “interested parties,” including not only the clients but others that need to verify lab practices, such as the regulatory and accreditation agencies. This terminology is already revised in the current ISO 9001 edition (4.2 of [8]).
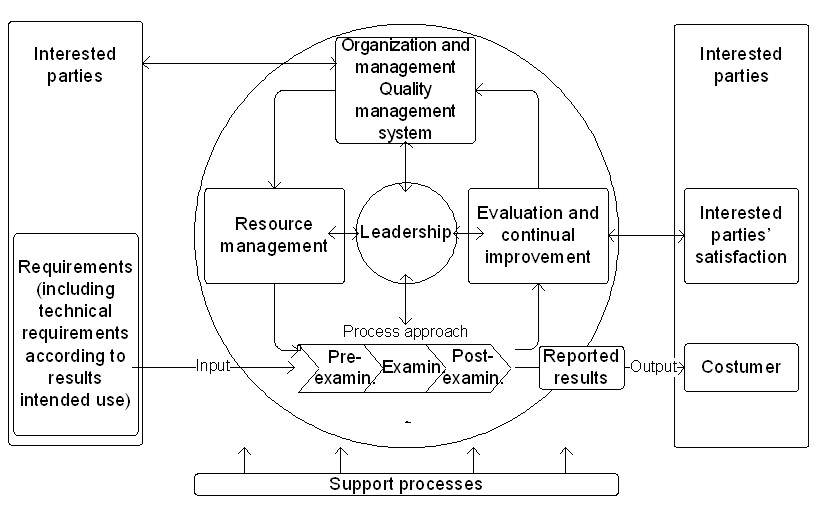
ISO 15189 technical requirements are applied for personnel, accommodation and environmental conditions, laboratory equipment, reagents, and consumables, pre-examination processes, examination processes, ensuring the quality of testing processes results, post-examination processes, reporting of results, the release of results, and laboratory information management. Table 1 summarizes these stipulations.
Table 1: Summary of ISO 15189 specifications
|
5.1 Personnel |
5.6 Ensuring quality of examination results |
|
Personnel qualifications documentation, job descriptions, personal introduction to the organizational environment program, training provision, competence assessment per person, reviews of staff performance, continuing education and professional development, and personal records of relevant skills. |
Quality control procedures design to verify the attainment of the intended quality of results, quality control materials, quality control data, interlaboratory comparisons, analysis of interlaboratory comparison samples, evaluation of laboratory performance, and comparability of examination results. |
|
5.2 Accommodation and environmental conditions |
5.7 Post-examination processes |
|
Laboratory and office facilities to provide an environment appropriate for the duties to be undertaken, storage facilities, staff services, patient sample collection facilities, facility maintenance, and environmental conditions. |
Review of results, storage, retention, and disposal of clinical samples. |
|
5.3 Laboratory equipment, reagents, and consumables |
5.8 Reporting of results |
|
Equipment: Documented procedure, acceptance testing, instructions for use, calibration and metrological traceability, maintenance and repair, adverse indented reporting, and records. Reagents and consumables: Documented procedure, reception and storage, acceptance testing, inventory management, instructions for use, adverse incident reporting, and records. |
Report of examination results, the report attributes, and content. |
|
5.4 Pre-examination processes |
5.9 Release of results |
|
Documented procedures, information for patients and users, request form information, first sample collection and handling, sample transportation, sample reception, pre-examination handling, preparation, and storage. |
Documented procedures, automatic selection and reporting of results, and revised reports. |
|
5.5 Examination processes |
5.10 Laboratory information management |
|
Examination procedure selection which has been validated for their intended use, verification or validation of tests, measurement uncertainty of measured quantity values, biological reference intervals or clinical decision values, and documentation of testing procedures. |
Authorities and responsibilities, and information system management. |
Sub-chapters 5.3, 5.5, and 5.6 require specifications for which there is not a harmonization of practices - note that all the results are recorded, and its traceability is assumed:
a) 5.3.1.4 Equipment calibration and metrological traceability
The medical laboratory participates in programs to calibrate and verification of trueness, i.e., to determine and verify bias (systematic error analysis) defined as “the difference between the expectation of the test results and an accepted reference value” (2.18 of [9]). Measurement Precision (random error analysis) is also measured and verified. It is defined as “the dispersion of independent results of measurements obtained under specific conditions, is expressed such as standard deviation or coefficient of variation”(2.15 of [9]). Preferably, traceable metrological materials should be used. When these materials are not available, or their use is not significant to the estimate accuracy, alternative materials could be used. See for a more in-depth discussion see [10-12].
b) 5.5 Examination processes require:
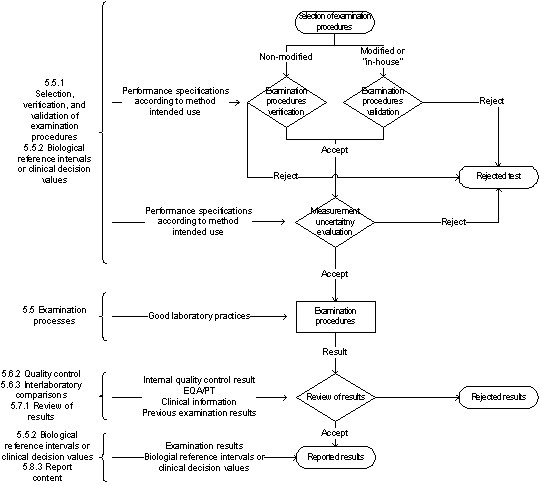
- 5.5.1.1 Selection of examination procedure: New tests are selected on the basis of clinical purpose (intended use, fit for purpose). For instance, a screening test selection in a blood bank should assure that a method with high diagnostic sensitivity [13] is chosen to minimize the residual risk (2.29 of [14]) of post-transfusion infection. ISO 15189does not recommend any approach to select a new test. Usually, it is based on a literature review using validation cases of state-of-the-art methods.
- 5.5.1.2 Verification of examination procedure: All tests used without modification - “non-waived” tests - are verified using performance information data available from the manufacturer. The verification shall confirm with evidence that the laboratory performance claims have been met. The calculations are based on experimental data. The methodology is presented in guidelines CLSI EP15-A3 [15] for quantitative tests or CLSI EP12-A2 [16] for qualitative tests.
- 5.5.1.3 Validation of examination procedure: Modified (“waved”) or “in-house” tests require a more complex validation process. Also, “standard methods used outside their intended scope” shall be validated. Again, these tests are validated according to the clinical test purpose/intended use/impact of the result on the clinical decision. Therefore, the specifications, such as the allowable total error, allowable diagnostic sensitivity or allowable diagnostic specificity are selected accordingly. ISO 15189 does not state any specific statistical approach for method validation, and it does not state any goals/targets/claims. As with verification of non-modified tests, experimental data is involved with validation of modified tests. The methodology is as complex as needed, and it is identical to what is required to the manufacturer in the validation phase. CLSI guidelines referred to in 5.5.1.2 can also be used, but further studies are needed. For example, the calculation of a clinical decision point based on representative samples of the population.
- 5.5.1.4 Measurement uncertainty of measured quantity values: This requirement is just for tests expressing quantitative results, as suggested by the subtitle. For qualitative results, measurement uncertainty cannot be computed. Even if the qualitative results are expressed on an ordinal scale according to cutoff, its determination is optional. ISO 15189 does not recommend a methodology to calculate measurement uncertainty. However, since ISO is a member of the working groups of the Guide to the uncertainty of measurement (GUM) [17] and the Vocabulary of international metrology (VIM) [9], it is presumed that an “Uncertainty approach” ([17]) model is mandatory. Empirical models should be used, preferably using data from the validation of the examination procedure phase. It is not recommended the use of external quality assessment (EQA)/proficiency of testing (PT) data as a primary source due to the heterogeneity of the results [18]. However, the EQA/PT is probably the most common model in medical laboratories with tests accreditated to ISO 15189. It does not comply with the standard since usually, this determination is not according to the results' intended use (clause 5.5.1.4 of [1]). ISO 15189 2nd edition required that measurement uncertainty is determined when its result was “relevant” and “possible” (5.6.2 of [4]) “Relevant” could be interpreted as when the outcome has a significant clinical value, and “possible” synonymous of the availability of a mathematical model. One such model available to labs is the total analytical error based on the “Error Approach” (also documented as Traditional Approach or True Value Approach). Measurement uncertainty is an additional model that is part of the evaluation of the quality of the reported result, which could be applied to any measurement. Target uncertainty must be defined, which can be very complex. See [19] for further details. Measurement uncertainty implementation has not been widely successful in medical laboratories, even after 23 years since GUM was published, such as demonstrated by the Westgard QC 2015 survey [20]. Note that the measurement uncertainty evaluation can be interpreted as redundancy of verification and validation, just differing due to the use of the “Uncertainty Approach.” For further details about models to determine measurement uncertainty in medical laboratories see [21].
c) 5.6 Ensuring quality of examination results
- Internal quality control: Design of internal quality control scheme is encouraged and implied, but no approach is recommended. Most of the methods used in labs are applications of the "Westgard Rules,” however just using a multirule QC approach does not automatically fulfill the principles [22], and around the world the practice of QC is not harmonized. An alternative to the Levey-Jennings charts could be used, such as the exponentially weighted moving average (EWMA) chart (9.2 of [23]). Nevertheless, the practical suggestion is to use statistical QC based on the Sigma-metrics [24] principally because it relates the determined total error with the allowable total error. The allowable total error is equivalent to the error that does not significantly contribute to incorrect clinical decisions. For a depth discussion on this issue, please see [25].
- External quality assessment: Medical laboratories shall participate in programs for EQA/PT and shall provide evidence of corrective actions when results are out of control. For instance, when a result is out of acceptable group requirements. There are no specific recommended approaches. The use of the EQA/PT approach is sometimes misunderstood, because some calculations based on this data may be inconsistent. For example, EQA/PT could be an unreliable source for estimates of bias if the peer group’s results have a large discrepancy.
Figure 2 represents the steps from the test selection to the reported results. The accomplishment of the examination and post-examination phases are dependent on the pre-examination stage.
Frequently-Asked Questions about ISO 15189
Which books are suggested to support the ISO 15189 quality management system?
Principally two publications: David Burnett, Ph.D. “A Practical guide to ISO 15189 in laboratory medicine” (2013), and James Westgard, Ph.D. and Sten Westgard, M.Sc. “Basic quality management systems” (2014).
Which references can support ISO 15189 specifications on examination and post-examination activities?
a) Method selection
See Westgard QC lesson no. 20 Selecting a method to validate and Basic method validation 3rd ed. (2008) book
b) Method verification and validation
Precision:
-Detection limit: See Westgard QC lesson no. 29 The detection limit experiment, Basic method validation 3rd ed. (2008) book, and CLSI EP17
-Precision components: See Westgard QC lesson no. 22 The replication experiment, Basic method validation 3rd ed. (2008) book, and CLSI EP15 (also EP5, EP9, and EP19)
-Bias: Proportional and constant bias: See Westgard QC lesson no. 23 The comparison of methods experiment, Basic method validation 3rd ed. (2008) book, and CLSI EP15 (also EP9 and EP10)
- Bias: Drift and carryover: See Basic method validation 3rd ed. (2008) book, and CLSI EP10
- Bias: Linearity: See Westgard QC lesson no. 26 The linearity or reportable range experiment, Basic method validation 3rd ed. (2008) book, and CLSI EP6 and EP10
- Bias: Interferences: See Westgard QC lesson no. 27 Interference and recovery experiments, Basic method validation 3rd ed. (2008) book, and CLSI EP7 and EP14
-Total error: See Westgard QC lesson no. 23 The comparison of methods experiment, Basic method validation 3rd ed. (2008) book, and CLSI EP21
-Qualitative assays: See Westgard QC essay Basic validation of qualitative tests, Statistical methods in diagnostic medicine. 2nd ed. (2011) book, and CLSI EP12 and EP24
b) Measurement uncertainty
-Modular approach: See Westgard QC essay Time to engage in MU, GUM, EURACHEM QUAM books, and CLSI EP29
-Empirical approach: S: See Westgard QC essay The Hitch-hiker’s guide to MU in clinical laboratories, Uncertainty of Measurement in Medical Laboratories chapter, EURACHEM QUAM, NordTest TR 537, and EURACHEM Target Uncertainty books, and CLSI EP29
c) Internal quality control
See Westgard QC lesson no. 74 Best practices for “Westgard rules”, Six Sigma quality design and control 2nd ed. (2006) book, and CLSI C24
d) External quality assessment/proficiency testing
See Westgard QC Quality Requirements, Six Sigma quality design and control 2nd ed. (2006) book, and CLSI GP27
e) Reference intervals
See Westgard QC essay FAQ in reference intervals and biological variation, Statistical bases of reference values in laboratory medicine (1995) book, and CLSI C28
f) Risk management
See Westgard QC Risk management essays, Six Sigma risk analysis (2011) book, and CLSI EP18 and EP23.
For the validation of examination, procedures are suggested Medcalc (MedCalc Software bvba), EP Evaluator (Data Innovations), and Analyse-it (Analyse-it Software, Ltd.). Dietmar Stöckl, Ph.D. offers a huge number of spreadsheets helpful to validation at STT Consulting. For measurement, uncertainty calculated is recommended by the MUKit (SYKE). This is a freeware based on [26]. For IQC there are many software programsavailable, some based on Web services. For an IQC statistical design based on Sigma-metrics is the legacy EZ Rules (Westgard QC), but also Bio-Rad's Westgard Advisor.
Is there some guideline based on audit requirements (4.13)?
Yes, ISO 19011:2018 [28] “is intended to apply to a broad range of potential users.” It is the recommendation to support the audits, including the documented procedure. Part 4 of these series will is oriented to audit requirements [13].
Is there some guideline to support safety specifications (5.2)?
Yes, ISO 15190:2003 [29] is the complementary standard to ISO 15189. Part 5 of these series is based on safety requirements.
What is happening with ISO 15189 implementation from a global perspective?
Currently, ISO 15189 is obligatory in Australia and Latvia. Since 2011 all new French medical laboratories must be accredited. All other public or private laboratories in France must be accredited after November 1, 2016, on at least 50% of the tests, 70% in 2018, and 100% in 2020. In the Netherlands, the CCKL accreditation has been changing to the ISO 15189 at the direction of the Dutch ‘Raad voor Accreditatie’ (RvA) after January 1st, 2018. Thus, the implementation case of ISO 15189 at a global perspective could be designated as unsuccessful, which is different from what is happening with ISO/IEC 17015 in other fields. On a harmonization perspective of good laboratory practices, this is a major concern.
Summary
Acreditation according to ISO 15180 has several advantages.
The pros could be summed up as:
- The only global standard for the accreditation of medical laboratory results;
- Based on good laboratory practices;
- Focus on technical specifications in the medical laboratory; - Process approach matching the pre-analytical, analytical, and post-analytical phases;
- Oriented to support accurate clinical decisions;
- Identification and traceability information of the different phases of the medical laboratory process;
- Monitoring and measuring of devices that significantly contribute to the trueness and uncertainty of the reported results;
- Training and competency assessment of the staff which is critical to good management and good laboratory practices, and;
- Infrastructure to correctly support operating practices.
Nevertheless, there are a few cons to ISO 15189:
- The accreditation is expensive when compared to the ISO 9001 certification;
- Its value is not well understood by the physician and the customers of clinical decisions;
- It is not used by most of the medical laboratory agencies as the standard to accreditation;
- It requires auditors with an advanced matrix of skills;
- It does not require sustainability;
- The specifications sometimes are too generic or abstract;
- It does not standardize critical practices such as the validation, measurement uncertainty, IQC and EQA/PT of examination procedures, and;
- The safety specifications are basic.
References
- International Organization for Standardization (2012). ISO 15189 Medical laboratories - Requirements for quality and competence. 3rd ed. Geneva: The Organization.
- International Organization for Standardization (2003). ISO 15189 Medical laboratories - Particular requirements for quality and competence. Geneva: The Organization.
- International Organization for Standardization (1999). ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories. Geneva: The Organization.
- International Organization for Standardization (2007). ISO 15189 Medical laboratories - Particular requirements for quality and competence. 2nd ed. Geneva: The Organization.
- Feigenbaum, A (1956). Total quality control. Harvard Bus Rev, 34(6):93-101.
- Deming, W (1982). Quality, productivity, and competence position. Cambridge (MA): Massachusetts Institute of Technology, Center for Advanced Study.
- Juran, J (1983). Upper management and quality. 4th ed. Wilton (CT): Juran Institute.
- International Organization for Standardization (2015). ISO 9001 Quality management systems - Requirements. 5th ed. Geneva: The Organization.
- Joint Committee for Guides in Metrology (2012). International Vocabulary of Metrology - Basic and General Concepts and Associated Terms. JCGM 200:2012, JCGM 200:2008 with minor corrections. JCGM
- EURACHEM/CITAC (2003). Traceability in chemical measurement. Europe: The Organizations. Retrieved from: http://www.eurachem.org/images/stories/Guides/pdf/EC_Trace_2003.pdf. Accessed: February 13, 2017.
- Clinical and Laboratory Standards Institute (2006). X-05R Metrological traceability and its implementation, A report. Wayne (PA): The Institute.
- Vesper H, Thienpont L (2009). Traceability in laboratory medicine. Clin Chem 55(6):1067-1075.
- Pereira P, Westgard J, Encarnação P, Seghatchian J (2015). Evaluation of the measurement uncertainty in screening immunoassays in blood establishments: Computation of diagnostic accuracy models. Transfus Apher Sci 52(1):35-41.
- International Organization for Standardization (2012). ISO 31000 Risk management - Principles and guidelines. Geneva: The Organization.
- Clinical and Laboratory Standards Institute (2014). EP15-A3 User verification of precision and estimation of bias. 3rd ed. Wayne (PA): CLSI.
- Clinical and Laboratory Standards Institute. EP12-A2 User protocol for evaluation of qualitative test performance. 2nd ed. Wayne (PA): CLSI, 2008.
- Joint Committee for Guides in Metrology (2008). Evaluation of measurement data - Guide to the expression of uncertainty in measurement. JCGM 100:2008, GUM 1995 with minor corrections. JCGM.
- Pereira P, Magnusson B, Theodorsson E, Westgard J, Encarnação P (2015). Measurement uncertainty as a tool for evaluating the ‘grey zone’ to reduce the false negatives in immunochemical screening of blood donors for infectious diseases. Accred Qual Assur 21:25-32.
- EURACHEM/CITAC (2015). Setting and Using Target Uncertainty in Chemical Measurement. Europe: The Organizations. Retrieved from: https://www.eurachem.org/images/stories/Guides/pdf/STMU_2015_EN.pdf. Accessed: February 13, 2017.
- Westgard QC (2016). MU Survey 2015: The Global Results. https://westgard.com/mu-global-survey.htm Accessed: February 13, 2017.
- Pereira P (2016). Uncertainty of measurement in medical laboratories. In book: Luigi Cocco (editor). New Trends and Developments in Metrology. Rijeka: InTech. Retrieved from: http://www.eurachem.org/images/stories/Guides/pdf/EC_Trace_2003.pdf. Accessed: February 13, 2017.
- Westgard J, Barry P, Hunt M, Groth T (1981). A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem 27(3):493-501.
- Montgomery D (2012). Introduction to statistical quality control. 7th ed. Hoboken (NJ): John Wiley & Sons, Inc.
- Westgard J (1992). Charts of operational process specifications (“OPSpecs charts”) for assessing the precision, accuracy, and quality control needed to satisfy proficiency testing performance criteria. Clin Chem 38:7:1226-1233.
- Westgard J (2006). Six Sigma Quality Design and Control. Madison (WI): Westgard QC.
- Magnusson B, Näykk T, Hovind H, Krysell M (2011). NordTest NT TR 537 Handbook for Calculation of Measurement Uncertainty in Environmental Laboratories. 3.1th ed. Oslo Nordic Innovation. Retrieved from: http://www.nordtest.info/images/documents/nt-technical-reports/nt_tr_537_ed3_1_English_Handbook%20for%20Calculation%20of%20Measurement%20uncertainty%20in%20environmental%20laboratories.pdf. Accessed: February 13, 2017.
- International Organization for Standardization. (2003). ISO 1012 Measurement management systems - Requirements for measurement processes and measuring equipment. Geneva: The Organization.
- International Organization for Standardization (2003). ISO 15190 Medical laboratories - Requirements for safety. Geneva: The Organization.
29. International Organization for Standardization (2018). ISO 19011 Guidelines for auditing management systems. 3rd ed. Geneva: The Organization.