Sigma Metric Analysis
Mindray BS 2000M in China, Multimode analysis
We look at the impact of new CLIA and EFLM goals on the assessment of a Mindray BS 2000M in China.
Mindray BS 2000M in China, multimode analysis
March 2023
Sten Westgard, MS
See the other analyses in this series:
- Beckman Coulter DxC 700
- Abbott Alinity
- Siemens Atellica
- Siemens Atellica in Romania
- Siemens Atellica in Spain
- Siemens ADVIA 2120i
- Roche c501 in Turkey
- Roche c501 in Saudi Arabia
- MicroLab RX-50 in India
- Roche cobas 6000 immunoassays in Turkey
- Sysmex XN 350 in India
- Mindray 7500 in China
- Mindray BS 2000M in China
This Mindray BS 2000M study comes from China:
Performance Evaluation Between Two Automated Biochemical Analyzer Systems: Roche Cobas 8000 and Mindray BS 2000M, Mingxing Chen, Simeng Qin, Sitao Yang, Huaping Chen, Liuyi Lu, Xue Qin, J Med Biochem 2022; 41(3). DOI: 10.5937/jomb0-34328
This study covers creatinine kinase (CK), lactate dehydrogenase-1 (LDH), retinol binding protein (RBP), cystatin-C, immuglobln A (IgA), immuglobin G (IgG), and immunoglobin M (IgM). We will calculate Sigma-metrics for all but RBP. It's a smaller group of tests than we usually like, but the authors selected these analytes because of their relationship to specific disease conditions.
"High, normal, and low control samples were run every day to monitor the sysem's performance according to National Laboratory Accreditation Board (NABL) and CLSI EP5-A3." Now, unfortunately for the reliability of the study, these controls were manufacturer controls. So if we look at sigma metrics calculated for these analytes, we must bear in mind these are probably optimistic. Independent controls are recommended by ISO 15189, CLSI C24, and other best practice guidelines.
I want to call a few erroneous statements to attention. "The coefficient of variation of quality control in all parameters was less than 10% which means that the results of quality control were in control." Having a CV less than 10% is a guarantee of nothing. Indeed, for many assays, a CV of 7, 8, or 9% would be unacceptable. "There was nothing unusual in control, which demonstrated that the quality of controls was acceptable." An absence of out-of-control events does not mean the control materials are acceptable. Indeed, manufacturer control materials, with the wrong QC settings, might very well produce an absence of flags, but that is only a false sense of security.
For Bias, "[a] total of 1869 remaining serum samples were collected from outpatients and inpatients at the Second Affiliated Hospital of Guangxi Medical University from July 2019 to Octobern 2019 for diagnostic accuracy.... After being tests on Cobas 8000 c702... those serum samples were immediately tested on Mindray BS2000M...Passing-Bablok regression analysis was used to evaluate the regression equation and the correlation of the two instruments." This is a very good number of samples and a better regression calculation. The comparison between Roche and Mindray is an interesting choice. The Roche assays are the stand-ins for reference methods.
The TEa goals applied can be found on our Consolidated Chemistry Performance Specifications page.
| MINDRAY BS2000M |
||
| TEST | % Bias | % CV |
| CK | 8.18 | 6.99 |
| LDH-1 | 15.19 | 4.83 |
| Cystatin-C | 37.07 | 5.84 |
| IgA | 4.06 | 3.40 |
| IgM | 2.83 | 4.43 |
| IgG | 18.27 | 2.81 |
Sigma-metrics according to EFLM-derived DESIRABLE performance specifications
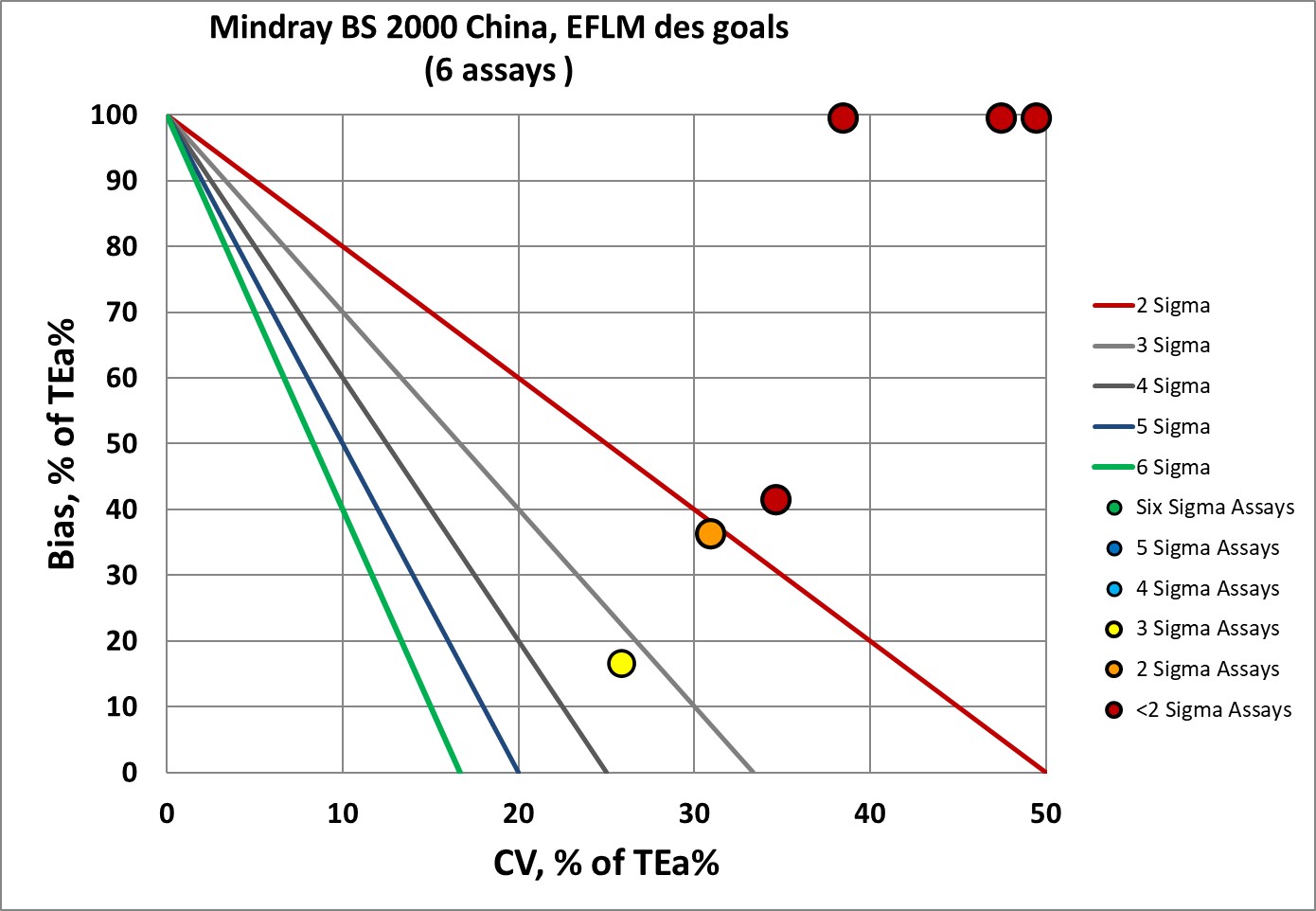
The EFLM desirable specifications used to be the de facto global standard, but have fallen out of favor due to their toughness.
The Mindray BS 2000M has four of six assays below 2 Sigma. Bias is off the charts for LDH-1, Cystatin-C and IgG. Imprecision is off the charts for LDH-1 and Cystatin-C as well. The best assay performance, for IgM, is only 3 Sigma.
Not surprising then, that EFLM recommended lowering the standards.
Sigma-metrics according to EuBIVAS-derived MINIMUM performance specifications
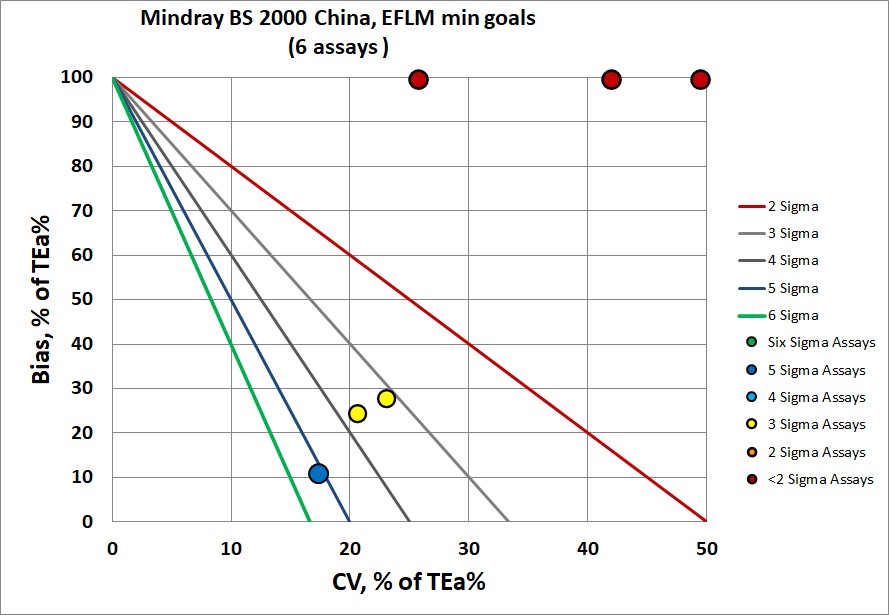
There is an improvement, but there is still half of the performance below 2 Sigma. The highlight assay, IgM, is 5.1.
Here's one of the most interesting new aspects of CLIA's 1992 and new 2024 goals. Are they more or less demanding than EFLM goals?
Sigma-metrics according to CLIA 1992 performance specifications
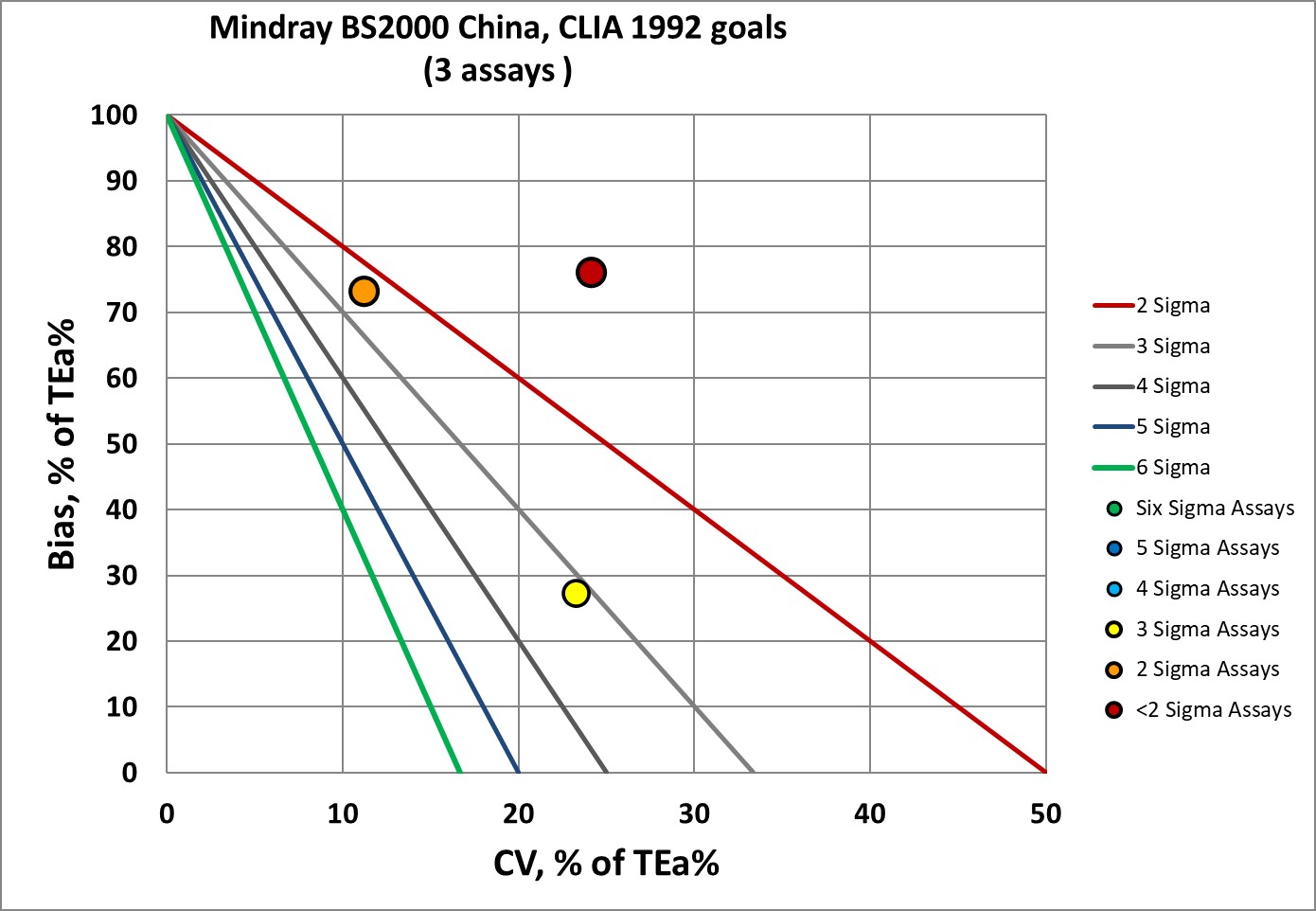
Now, most of the assays of the study are not covered by CLIA 1992. We can only plot 3 assays, CK, LDH-1, IgG, and none of them are good.
Sigma-metrics according to CLIA 2024 performance specifications
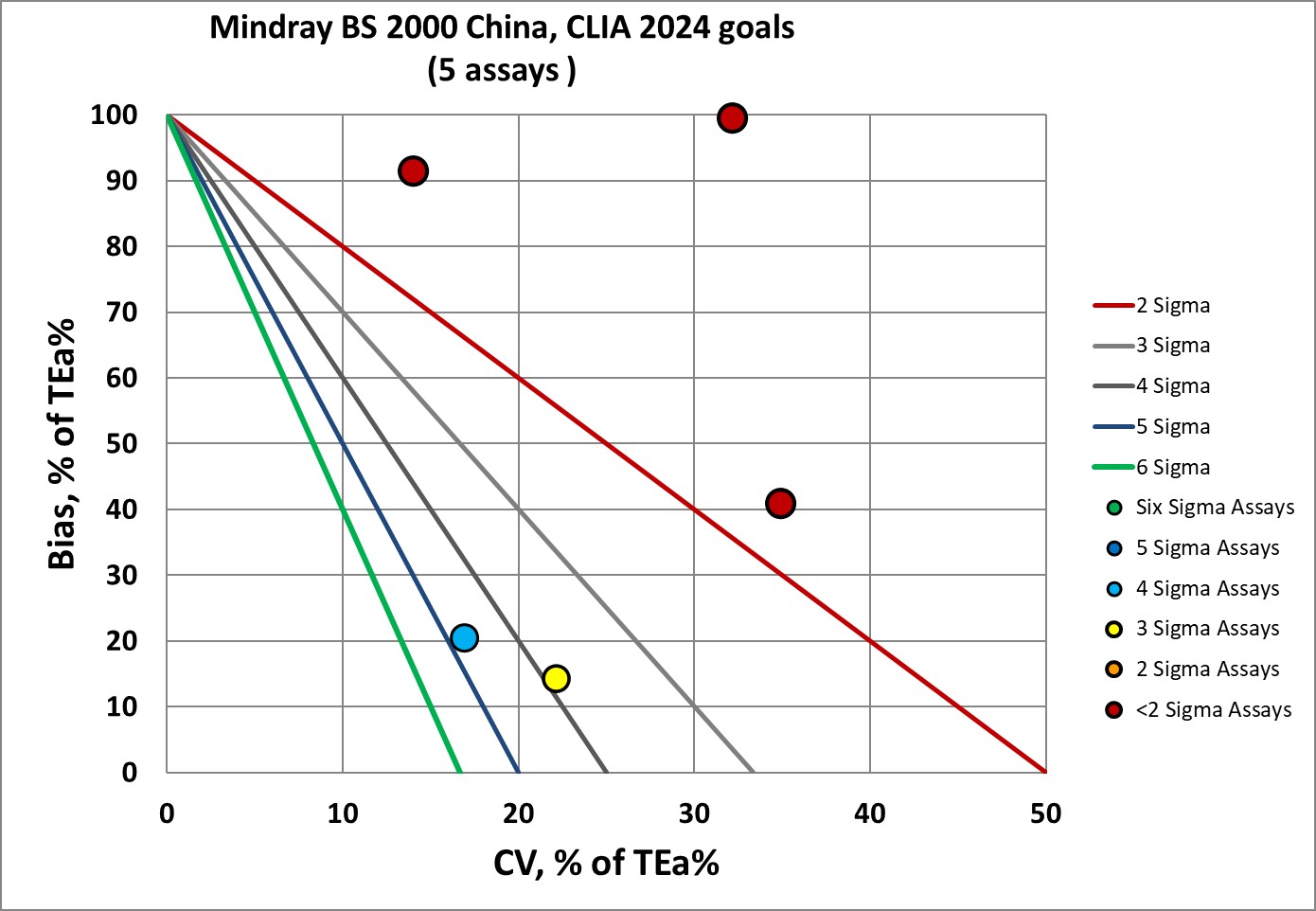
The CLIA 2024 goals cover more assays, so we're now looking at CK, LDH-1, Cystatin-C, IgA, IgM, and IgG. But the 2024 goals are more stringent than CLIA 1992, obviously. The verdict given by CLIA 2024 is almost the same as EFLM minimum specifications: 3 assays below 2 Sigma, 1 assay at 3 Sigma, and 1 assay at 4 Sigma. EFLM minimum specifications just give one assay a 5 Sigma, not 4 Sigma. So CLIA 2024 proves again that it is more demanding than EFLM minimum.
Conclusion
Even with possibly the most favorable assessment (from their own controls), the Mindray BS 2000M is not looking good. In optimal conditions, the performance, when judged by multiple standards, is unacceptable.
